122-11-2
| Name | sulfadimethoxine |
|---|---|
| Synonyms |
sulfadimethoxine
-N-((2,6-Dimethoxy-4-pyrimidinyl)sulfanilamide (8CI) T6N CNJ BO1 DO1 FMSWR DZ MFCD00057345 4-Amino-N-(2,6-dimethoxy-4-pyrimidinyl)benzenesulfonamide 4-Amino-N-[2,6-bis(methyloxy)pyrimidin-4-yl]benzenesulfonamide 4-amino-N-(2,6-dimethoxypyrimidin-4-yl)benzenesulfonamide EINECS 204-523-7 sulphadimethoxine |
| Description | Sulfadimethoxine is a sulfonamide antibiotic.Target: AntibacterialSulfadimethoxine is a sulfonamide antibiotic. Sulfadimethoxine is used to treat many infections including treatment of respiratory, urinary tract, enteric, and soft tissue infections. It is most frequently used in veterinary medicine, although it is approved in some countries for use in humans. Sulfadimethoxine inhibits bacterial synthesis of folic acid (pteroylglutamic acid) from para-aminobenzoic acid. Sulfadimethoxine is approved in Russia for use in humans, including children, and has been successfully used there for more than 35 years. It is widely available in Russia as an over-the-counter drug manufactured by a number of Russian pharmaceutical companies [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 548.5±60.0 °C at 760 mmHg |
| Melting Point | 200 °C |
| Molecular Formula | C12H14N4O4S |
| Molecular Weight | 310.329 |
| Flash Point | 285.5±32.9 °C |
| Exact Mass | 310.073578 |
| PSA | 124.81000 |
| LogP | 1.48 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.623 |
| Storage condition | 2-8°C |
| Water Solubility | NH4OH 1 M: 50 mg/mL, clear, faintly yellow |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H317-H319-H335 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36/37-S24/25-S23 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | WO9030000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2935009090 |
|
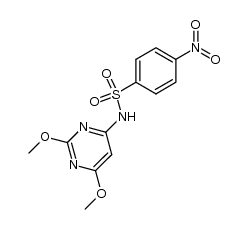
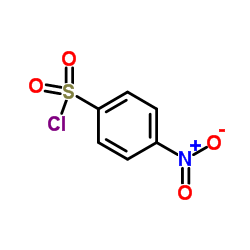
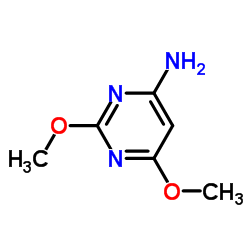
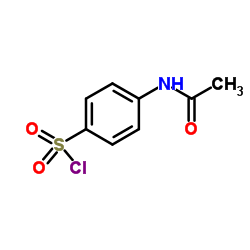
~79% 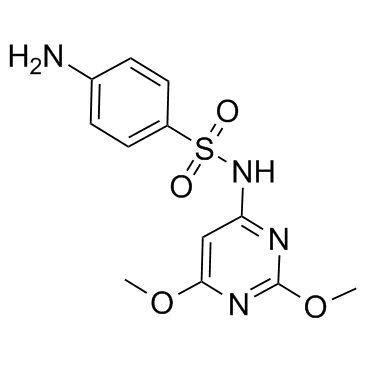
122-11-2 |
| Literature: Luk'yanov, A. V.; Onoprienko, V. S.; Borodina, K. S.; Zasosov, V. A.; Van'kovich, E. V.; et al. Pharmaceutical Chemistry Journal, 1980 , vol. 14, # 9 p. 640 - 644 Khimiko-Farmatsevticheskii Zhurnal, 1980 , vol. 14, # 9 p. 87 - 91 |
|
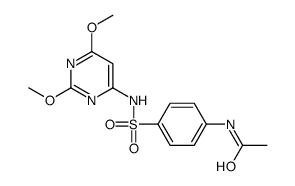
~% 
122-11-2 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 14, # 9 p. 640 - 644 Khimiko-Farmatsevticheskii Zhurnal, , vol. 14, # 9 p. 87 - 91 |
|
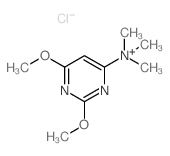
~% 
122-11-2 |
| Literature: Pharmaceutical Chemistry Journal, , vol. 14, # 9 p. 640 - 644 Khimiko-Farmatsevticheskii Zhurnal, , vol. 14, # 9 p. 87 - 91 |
|
~% 
122-11-2 |
| Literature: Monatshefte fuer Chemie, , vol. 87, p. 136,142 |
|
~% 
122-11-2 |
| Literature: Monatshefte fuer Chemie, , vol. 87, p. 136,142 |
|
~% 
122-11-2 |
| Literature: Monatshefte fuer Chemie, , vol. 87, p. 136,142 |
|
~% 
122-11-2 |
| Literature: Monatshefte fuer Chemie, , vol. 87, p. 136,142 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |