5116-24-5
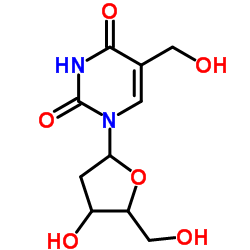
| Name | 5-hydroxymethyl-2'-deoxyuridine |
|---|---|
| Synonyms |
1-(2-Deoxypentofuranosyl)-5-(hydroxymethyl)-2,4(1H,3H)-pyrimidinedione
5-HMdU HMdUdr 1-(2-deoxypentofuranosyl)-5-(hydroxymethyl)pyrimidine-2,4(1H,3H)-dione 5-HYDROXYMETHYL-2'-DEOXYURIDINE |
| Description | 5-Hydroxymethyl-2'-deoxyuridine is a nucleoside analog. 5-Hydroxymethyl-2'-deoxyuridine inhibits the replication of multiple human leukemia cell lines with IC50 values of 1.7-5.8 μM. 5-Hydroxymethyl-2'-deoxyuridine prolongs the survival of mice carrying L1210 leukemia. 5-Hydroxymethyl-2'-deoxyuridine can be used for the research of cell replication and leukemia[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | 5-Hydroxymethyl-2'-deoxyuridine (0-10 μM) inhibits the replication of Sarcoma 180 cells and Ehrlich ascites carcinoma cells with ED50 values of 8.5 and 4 μM, respectively[1]. 5-Hydroxymethyl-2'-deoxyuridine inhibits herpes simplex virus type 1 (HSV-1) pyrimidine 2’-deoxyribonucleoside kinase with a Ki value of 3.5 μM[1]. 5-Hydroxymethyl-2'-deoxyuridine inhibits the replication of multiple human leukemia cell lines with IC50 values of 1.7-5.8 μM[2]. 5-Hydroxymethyl-2'-deoxyuridine (10-100 μM) shows dose-dependent toxicity against a human acute promyelocytic leukemia cell line[2]. |
| In Vivo | 5-Hydroxymethyl-2'-deoxyuridine (0, 5 and 50 mg/kg; i.p.; once) increases the survival of DBA/2 mice carrying L1210 leukemia[3]. |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 401.48°C (rough estimate) |
| Melting Point | 176-179 °C |
| Molecular Formula | C10H14N2O6 |
| Molecular Weight | 258.228 |
| Exact Mass | 258.085175 |
| PSA | 124.78000 |
| LogP | -1.49 |
| Index of Refraction | 1.610 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |