CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
UU5270000
-
CHEMICAL NAME :
-
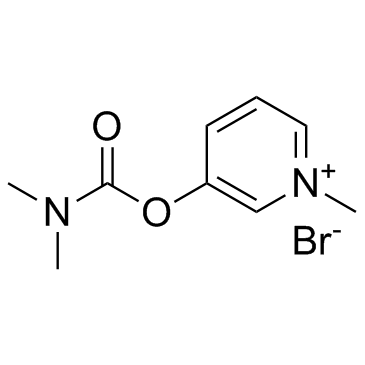
Pyridinium, 3-hydroxy-1-methyl-, bromide, dimethylcarbamate (ester)
-
CAS REGISTRY NUMBER :
-
101-26-8
-
LAST UPDATED :
-
199806
-
DATA ITEMS CITED :
-
20
-
MOLECULAR FORMULA :
-
C9-H13-N2-O2.Br
-
MOLECULAR WEIGHT :
-
261.15
-
WISWESSER LINE NOTATION :
-
T6KJ A1 COVN1&1 &E
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
7800 ug/kg
-
TOXIC EFFECTS :
-
Peripheral Nerve and Sensation - fasciculations Sense Organs and Special Senses (Eye) - visual field changes Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
IJMDAI Israel Journal of Medical Sciences. (POB 1435, Jerusalem 91013, Israel) V.1- 1965- Volume(issue)/page/year: 27,659,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
9 mg/kg
-
TOXIC EFFECTS :
-
Peripheral Nerve and Sensation - fasciculations
-
REFERENCE :
-
IJMDAI Israel Journal of Medical Sciences. (POB 1435, Jerusalem 91013, Israel) V.1- 1965- Volume(issue)/page/year: 27,659,1991
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
37500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,1042,1995
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2699 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 4,S195,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3100 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JMCMAR Journal of Medicinal Chemistry. (American Chemical Soc., Distribution Office Dept. 223, POB POB 57136, West End Stn., Washington, DC 20037) V.6- 1963- Volume(issue)/page/year: 26,145,1983
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2790 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DCTODJ Drug and Chemical Toxicology. (Marcel Dekker, 270 Madison Ave., New York, NY 10016) V.1- 1977/78- Volume(issue)/page/year: 7,507,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
16 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - miosis (pupillary constriction) Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory stimulation
-
REFERENCE :
-
GNRIDX Gendai no Rinsho. (Tokyo, Japan) V.1-10, 1967-76(?). Volume(issue)/page/year: 2,828,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,354,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1500 ug/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - miosis (pupillary constriction) Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory stimulation
-
REFERENCE :
-
GNRIDX Gendai no Rinsho. (Tokyo, Japan) V.1-10, 1967-76(?). Volume(issue)/page/year: 2,828,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
MECHAN Medicinal Chemistry, A Series of Reviews. (New York, NY) V.1-6, 1951-63. Discontinued. Volume(issue)/page/year: 3,329,1956 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1350 mg/kg/15D-C
-
TOXIC EFFECTS :
-
Blood - other changes Musculoskeletal - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - true cholinesterase
-
REFERENCE :
-
TOPADD Toxicologic Pathology. (c/o Dr. F.A. de la Iglesia, Warner-Lambert Co., Pharmaceutical Research Div., POB 1047, Ann Arbor, MI 48106) V.6(3/4)- 1978- Volume(issue)/page/year: 18,387,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
140 mg/kg/14D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from small intestine Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - true cholinesterase Related to Chronic Data - death
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 14,40,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
168 mg/kg/28D-I
-
TOXIC EFFECTS :
-
Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - true cholinesterase
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 14,40,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
540 mg/kg/90D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - true cholinesterase
-
REFERENCE :
-
FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 14,40,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Bird - chicken
-
DOSE/DURATION :
-
225 mg/kg/9W-I
-
TOXIC EFFECTS :
-
Blood - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - true cholinesterase
-
REFERENCE :
-
JTEHD6 Journal of Toxicology and Environmental Health. (Hemisphere Pub., 1025 Vermont Ave., NW, Washington, DC 20005) V.1- 1975/76- Volume(issue)/page/year: 48,35,1996 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
630 mg/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
TXCYAC Toxicology. (Elsevier Scientific Pub. Ireland, Ltd., POB 85, Limerick, Ireland) V.1- 1973- Volume(issue)/page/year: 69,291,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
280 mg/kg
-
SEX/DURATION :
-
female 15-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
TXCYAC Toxicology. (Elsevier Scientific Pub. Ireland, Ltd., POB 85, Limerick, Ireland) V.1- 1973- Volume(issue)/page/year: 69,291,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
TXCYAC Toxicology. (Elsevier Scientific Pub. Ireland, Ltd., POB 85, Limerick, Ireland) V.1- 1973- Volume(issue)/page/year: 69,291,1991 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 81490 No. of Facilities: 58 (estimated) No. of Industries: 1 No. of Occupations: 2 No. of Employees: 230 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 81490 No. of Facilities: 46 (estimated) No. of Industries: 1 No. of Occupations: 2 No. of Employees: 1619 (estimated) No. of Female Employees: 791 (estimated)
|