37936-89-3
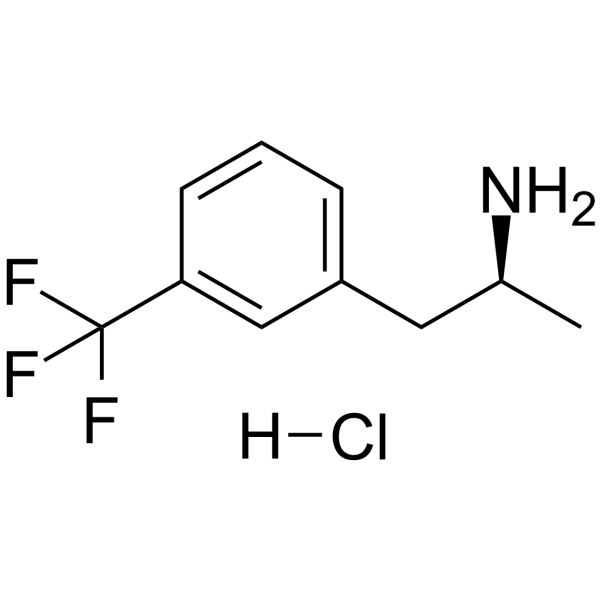
| Name | (2S)-1-[3-(trifluoromethyl)phenyl]propan-2-amine,hydrochloride |
|---|
| Description | (+)-Norfenfluramine hydrochloride, a major hepatic metabolite of (+)-fenfluramine, is a selective 5-HT2B receptor agonist (Ki: 11.2 nM). (+)-Norfenfluramine hydrochloride potently stimulates the hydrolysis of inositol phosphates and increases intracellular Ca2+. (+)-Norfenfluramine hydrochloride can be used for the research of primary pulmonary hypertension and valvular heart disease[1]. |
|---|---|
| Related Catalog | |
| Target |
5-HT2B Receptor:11.2 nM (Ki) 5-HT2C Receptor:324 nM (Ki) 5-HT2A Receptor:1516 nM (Ki) |
| In Vitro | (+)-Norfenfluramine (1 nM to 100 μM) hydrochloride contracts arteries with a dramatic decrease in threshold (aorta and mesenteric resistance artery) in rats[1]. (+)-Norfenfluramine (1 and 10 μM, 3 min) hydrochloride induces contraction in aorta from tissues of normotensive and hypertensive rats[1]. (+)-Norfenfluramine (0-10 μM, 3 min) hydrochloride induces 5-HT release from rat hippocampal synaptosomes by Ca2+-dependent way [2]. |
| In Vivo | (+)-Norfenfluramine (1-300 μg/kg, i.v.) hydrochloride induces pressor response in conscious SHAM and DOCA-salt rats[1]. (+)-Norfenfluramine (2.5 and 5 mg/kg, i.p.) hydrochloride decreases of 5-HT and 5-HIAA levels in telencephalon and brainstem of rats[3]. Animal Model: Conscious SHAM and DOCA-salt rats[1]. Dosage: 1-300 μg/kg Administration: Intravenous injection (i.v.), given in a cumulative fashion at 6-min intervals. Result: Induced pressor response in conscious SHAM and DOCA-salt rats. (change in mean arterial blood pressure at 300 μg/kg, mm Hg, SHAM vehicle=36, SHAM ketanserin=7, DOCA=51, DOCA ketanserin=19). |
| References |
| Molecular Formula | C10H13ClF3N |
|---|---|
| Molecular Weight | 239.66500 |
| Exact Mass | 239.06900 |
| PSA | 26.02000 |
| LogP | 4.09740 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| RIDADR | NONH for all modes of transport |