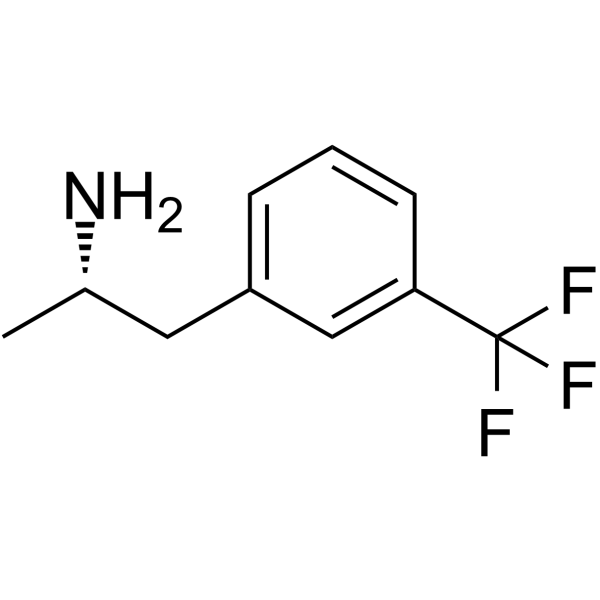
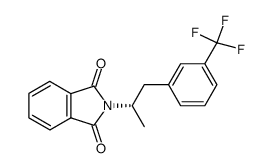
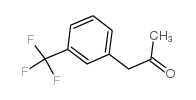
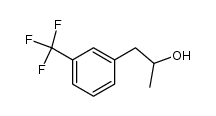
19036-73-8
| Name | (s)-1-(3-trifluoromethylphenyl)-2-aminopropane |
|---|---|
| Synonyms |
dexnorfenfluramine
Dexibuprofen Lysine d-norfenfluramine |
| Description | (+)-Norfenfluramine a major hepatic metabolite of (+)-fenfluramine, is a selective 5-HT2B receptor agonist (Ki: 11.2 nM). (+)-Norfenfluramine potently stimulates the hydrolysis of inositol phosphates and increases intracellular Ca2+. (+)-Norfenfluramine can be used for the research of primary pulmonary hypertension and valvular heart disease[1]. |
|---|---|
| Related Catalog | |
| Target |
5-HT2B Receptor:11.2 nM (Ki) 5-HT2A Receptor:1516 nM (Ki) 5-HT2C Receptor:324 nM (Ki) |
| In Vitro | (+)-Norfenfluramine (1 nM to 100 μM) contracts arteries with a dramatic decrease in threshold (aorta and mesenteric resistance artery) in rats[1]. (+)-Norfenfluramine (1 and 10 μM, 3 min) induces contraction in aorta from tissues of normotensive and hypertensive rats[1]. (+)-Norfenfluramine (0-10 μM, 3 min) induces 5-HT release from rat hippocampal synaptosomes by Ca2+-dependent way [2]. |
| In Vivo | (+)-Norfenfluramine (1-300 μg/kg, i.v.) induces pressor response in conscious SHAM and DOCA-salt rats[1]. (+)-Norfenfluramine (2.5 and 5 mg/kg, i.p.) decreases of 5-HT and 5-HIAA levels in telencephalon and brainstem of rats[3]. Animal Model: Conscious SHAM and DOCA-salt rats[1]. Dosage: 1-300 μg/kg Administration: Intravenous injection (i.v.), given in a cumulative fashion at 6-min intervals. Result: Induced pressor response in conscious SHAM and DOCA-salt rats. (change in mean arterial blood pressure at 300 μg/kg, mm Hg, SHAM vehicle=36, SHAM ketanserin=7, DOCA=51, DOCA ketanserin=19). |
| References |
| Density | 1.152g/cm3 |
|---|---|
| Boiling Point | 215.2ºC at 760mmHg |
| Molecular Formula | C10H12F3N |
| Molecular Weight | 203.20400 |
| Flash Point | 88.9ºC |
| Exact Mass | 203.09200 |
| PSA | 26.02000 |
| LogP | 3.29540 |
| Vapour Pressure | 0.15mmHg at 25°C |
| Index of Refraction | 1.467 |
| HS Code | 2921499090 |
|---|
| Precursor 4 | |
|---|---|
| DownStream 0 | |
| HS Code | 2921499090 |
|---|---|
| Summary | 2921499090 other aromatic monoamines and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |