13408-56-5
| Name | ponasterone A |
|---|---|
| Synonyms |
25-Deoxyecdysterone
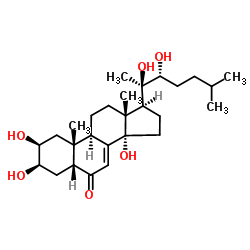
(2b,3b,5b,22R)-2,3,14,20,22-Pentahydroxycholest-7-en-6-one ponasterone A (2β,3β,5β,22R)-2,3,14,20,22-Pentahydroxycholest-7-en-6-one 25-Deoxy-20-hydroxyecdysone MFCD00272144 (2S,3R,5R,9R,10R,13R,14S,17S)-17-[(2R,3R)-2,3-dihydroxy-6-methylheptan-2-yl]-2,3,14-trihydroxy-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one |
| Description | Ponasterone A (25-Deoxyecdysterone), an ecdysteroid, has strong affinity for the ecdysone receptor. Ponasterone A is a potent regulator of gene expression in cells and transgenic animals, enabling reporter genes to be turned on and off rapidly[1][2]. |
|---|---|
| Related Catalog | |
| Target |
ecdysone receptor[2] |
| In Vitro | Ponasterone A (1 nM-100 μM) shows significant gene induction activity in CV-1 cells[2]. |
| In Vivo | Ponasterone A (3-10 mg; i.p.) produces robust inductions of gene expression in transactivator and reporter mice[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 640.5±55.0 °C at 760 mmHg |
| Molecular Formula | C27H44O6 |
| Molecular Weight | 464.635 |
| Flash Point | 355.2±28.0 °C |
| Exact Mass | 464.313782 |
| PSA | 118.22000 |
| LogP | 1.55 |
| Vapour Pressure | 0.0±4.3 mmHg at 25°C |
| Index of Refraction | 1.582 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |