23526-45-6
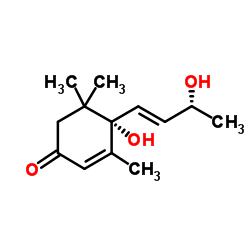
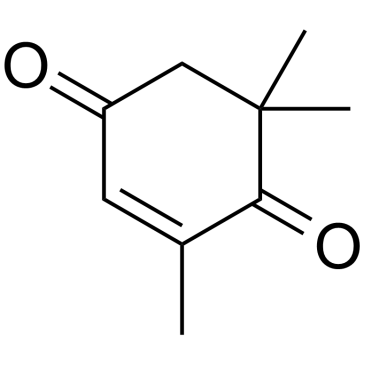
| Name | (6S,9R)-vomifoliol |
|---|---|
| Synonyms |
L6V BUTJ C1 DQ D1U1YQ1 E1 E1 &&(4S)-(1E,3R)- Form
(4S)-4-hydroxy-4-[(E,3R)-3-hydroxybut-1-enyl]-3,5,5-trimethylcyclohex-2-en-1-one Blumenol A (4S)-4-hydroxy-4-[(1E,3R)-3-hydroxybut-1-en-1-yl]-3,5,5-trimethylcyclohex-2-en-1-one Roseoside aglycon (6S,9R)-vomifoliol UNII:B7QV234K84 Vomifoliol (4S)-4-Hydroxy-4-[(1E,3R)-3-hydroxy-1-buten-1-yl]-3,5,5-trimethyl-2-cyclohexen-1-one |
| Description | Vomifoliol, a compound related to abscisie acid (ABA), has a modified 2,4-pentadiene side chain and has activity equal to that displayed by ABA. Vomifoliol exhibits antiacetylcholinesterase activity and displays moderate antileishmanial activity[1][2]. |
|---|---|
| Related Catalog | |
| References |
[1]. Stuart KL, et al. The effect of vomifoliol on stomatal aperture. Planta. 1975;122(3):307-310. |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 362.3±42.0 °C at 760 mmHg |
| Molecular Formula | C13H20O3 |
| Molecular Weight | 224.296 |
| Flash Point | 187.1±24.4 °C |
| Exact Mass | 224.141251 |
| PSA | 57.53000 |
| LogP | 1.05 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.567 |
| Hazard Codes | Xi |
|---|
| Precursor 0 | |
|---|---|
| DownStream 9 | |