N-Acetyl-L-cysteine-d3
Modify Date: 2024-01-02 15:05:11
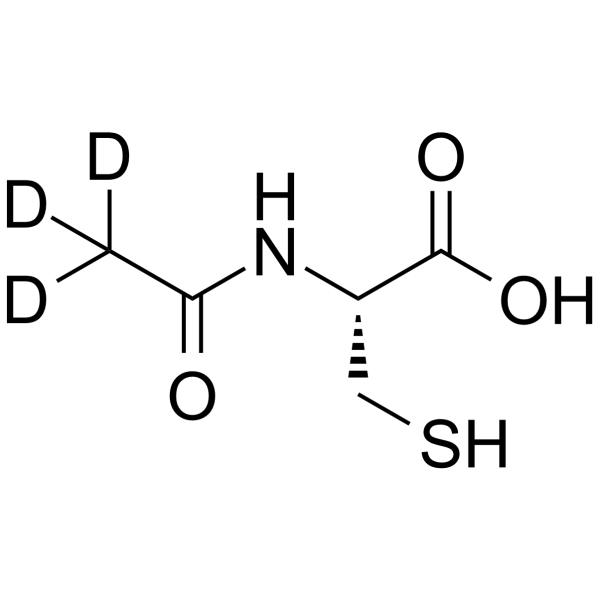
N-Acetyl-L-cysteine-d3 structure
|
Common Name | N-Acetyl-L-cysteine-d3 | ||
|---|---|---|---|---|
| CAS Number | 131685-11-5 | Molecular Weight | 166.21300 | |
| Density | 1.319 g/cm3 | Boiling Point | 407.678ºC at 760 mmHg | |
| Molecular Formula | C5H6D3NO3S | Melting Point | 98-100ºC | |
| MSDS | N/A | Flash Point | 200.357ºC | |
Use of N-Acetyl-L-cysteine-d3Acetylcysteine-d3 (N-Acetylcysteine-d3) is the deuterium labeled Acetylcysteine. Acetylcysteine (N-Acetylcysteine) is a mucolytic agent which reduces the thickness of the mucus. Acetylcysteine is a ROS inhibitor[1]. Acetylcysteine is a cysteine precursor, prevents hemin-induced ferroptosis by neutralizing toxic lipids generated by arachidonate-dependent activity of 5-lipoxygenases[5]. Acetylcysteine induces cell apoptosis[2][3]. Acetylcysteine also has anti-influenza virus activities[7]. |
| Name | N-Acetyl-L-cysteine-d3 |
|---|---|
| Synonym | More Synonyms |
| Description | Acetylcysteine-d3 (N-Acetylcysteine-d3) is the deuterium labeled Acetylcysteine. Acetylcysteine (N-Acetylcysteine) is a mucolytic agent which reduces the thickness of the mucus. Acetylcysteine is a ROS inhibitor[1]. Acetylcysteine is a cysteine precursor, prevents hemin-induced ferroptosis by neutralizing toxic lipids generated by arachidonate-dependent activity of 5-lipoxygenases[5]. Acetylcysteine induces cell apoptosis[2][3]. Acetylcysteine also has anti-influenza virus activities[7]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Density | 1.319 g/cm3 |
|---|---|
| Boiling Point | 407.678ºC at 760 mmHg |
| Melting Point | 98-100ºC |
| Molecular Formula | C5H6D3NO3S |
| Molecular Weight | 166.21300 |
| Flash Point | 200.357ºC |
| Exact Mass | 166.04900 |
| PSA | 105.20000 |
| Index of Refraction | 1.519 |
| Storage condition | -20°C Freezer |
| (2R)-3-sulfanyl-2-[(2,2,2-trideuterioacetyl)amino]propanoic acid |