Organic Letters
2004-02-05
Diastereoselective additions of chiral vinylzinc reagents to alpha-chiral aldehydes.
James A Marshall, Patrick Eidam
Index: Org. Lett. 6(3) , 445-8, (2004)
Full Text: HTML
Abstract
[reaction: see text] Additions of vinylic zinc bromide reagents to alpha-chiral aldehydes (R(1) = CH(2)OTBS, R(2) = Me; R(1) = Me, R(2) = OTBS) in the presence of lithiated (+)- or (-)-N-methylephedrine proceed with predominant reagent control to afford anti or syn adducts stereoselectively, except when the aldehydes possess an alkoxy substituent at the alpha- or beta-positions (R(1) = Me, R(2) = OBn; R(1) = CH(2)OBn, R(2) = Me), in which case chelation-controlled adducts predominate.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
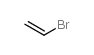 |
bromoethene
CAS:593-60-2 |
C2H3Br |
Related Articles:
More...
|
Reactivity in nucleophilic vinylic substitution (S(N)V):S(N)...
2013-09-06 [J. Org. Chem. 78(17) , 8574-84, (2013)] |
|
Asymmetric synthesis of enantioenriched (+)-elaeokanine A.
2006-07-21 [J. Org. Chem. 71(15) , 5674-8, (2006)] |
|
Formation of enehydrazine intermediates through coupling of ...
2013-01-21 [Angew. Chem. Int. Ed. Engl. 52(4) , 1266-9, (2013)] |
|
Total synthesis of polycavernoside A, a lethal toxin of the ...
2005-07-08 [J. Org. Chem. 70(14) , 5449-60, (2005)] |
|
Synthesis of the C1-C13 tetraenoate subunit of the chivosazo...
2010-08-20 [Org. Lett. 12(16) , 3724-7, (2010)] |