bromoethene
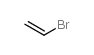
bromoethene structure
|
Common Name | bromoethene | ||
|---|---|---|---|---|
| CAS Number | 593-60-2 | Molecular Weight | 106.94900 | |
| Density | 1.517 g/mL at 25 °C(lit.) | Boiling Point | 16 °C750 mm Hg(lit.) | |
| Molecular Formula | C2H3Br | Melting Point | −139 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 1 °F | |
| Symbol |



GHS02, GHS07, GHS08 |
Signal Word | Danger | |
| Name | bromoethene |
|---|---|
| Synonym | More Synonyms |
| Density | 1.517 g/mL at 25 °C(lit.) |
|---|---|
| Boiling Point | 16 °C750 mm Hg(lit.) |
| Melting Point | −139 °C(lit.) |
| Molecular Formula | C2H3Br |
| Molecular Weight | 106.94900 |
| Flash Point | 1 °F |
| Exact Mass | 105.94200 |
| LogP | 1.52480 |
| Vapour density | 3.8 (15 °C, vs air) |
| Vapour Pressure | 1551 mm Hg ( 37.8 °C) |
| Index of Refraction | n20/D 1.410 |
| Stability | Stable, but may polymerize in sunlight. Reacts violently with all types of oxidizer. Incompatible with strong oxidizing agents, peroxides, copper, copper alloys, plastics. Highly flammable. |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302-H319-H335-H350 |
| Supplemental HS | May form explosive peroxides. |
| Precautionary Statements | P201-P210-P280-P308 + P313-P370 + P378-P403 + P235 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | F+:Highlyflammable;T:Toxic; |
| Risk Phrases | R45;R12 |
| Safety Phrases | S53-S9-S16-S29-S33-S45 |
| RIDADR | UN 1993 3/PG 1 |
| WGK Germany | 3 |
| RTECS | KU8400000 |
| Hazard Class | 2.1 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
|
Reactivity in nucleophilic vinylic substitution (S(N)V):S(N)Vπ versus S(N)Vσ mechanistic dichotomy.
J. Org. Chem. 78(17) , 8574-84, (2013) The intrinsic electronic factors that determine reactivity in prototypical identity nucleophilic vinylic substitution reactions, X(-) + ViX → XVi + X(-) (Vi = vinyl), have been studied by performing q... |
|
|
Asymmetric synthesis of enantioenriched (+)-elaeokanine A.
J. Org. Chem. 71(15) , 5674-8, (2006) The key transformation in the total synthesis of (+)-elaeokanine A was accomplished by asymmetric deprotonation of N-Boc pyrrolidine, followed by the reaction of the in situ generated enantioenriched ... |
|
|
Formation of enehydrazine intermediates through coupling of phenylhydrazines with vinyl halides: entry into the Fischer indole synthesis.
Angew. Chem. Int. Ed. Engl. 52(4) , 1266-9, (2013)
|
| Vinyl bromide |
| MFCD00000183 |
| cis-vinyl bromide |
| trans vinyl bromide |
| bromoethylene |
| EINECS 209-800-6 |
 CAS#:106-93-4
CAS#:106-93-4 CAS#:74-86-2
CAS#:74-86-2 CAS#:4170-50-7
CAS#:4170-50-7 CAS#:557-91-5
CAS#:557-91-5 CAS#:54509-73-8
CAS#:54509-73-8 CAS#:57718-12-4
CAS#:57718-12-4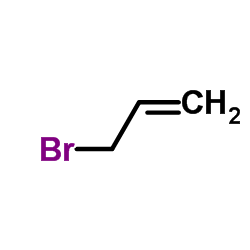 CAS#:106-95-6
CAS#:106-95-6 CAS#:61853-37-0
CAS#:61853-37-0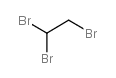 CAS#:78-74-0
CAS#:78-74-0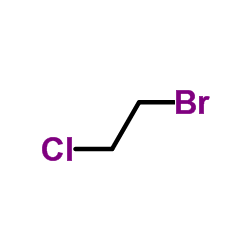 CAS#:107-04-0
CAS#:107-04-0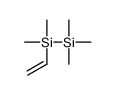 CAS#:1112-06-7
CAS#:1112-06-7 CAS#:4393-06-0
CAS#:4393-06-0![Benzene,[1-[(trimethylsilyl)oxy]-2-propen-1-yl]- structure](https://image.chemsrc.com/caspic/046/19917-00-1.png) CAS#:19917-00-1
CAS#:19917-00-1 CAS#:3485-84-5
CAS#:3485-84-5 CAS#:471-25-0
CAS#:471-25-0 CAS#:142-45-0
CAS#:142-45-0 CAS#:405-99-2
CAS#:405-99-2 CAS#:398-23-2
CAS#:398-23-2
