| Structure | Name/CAS No. | Articles |
|---|---|---|
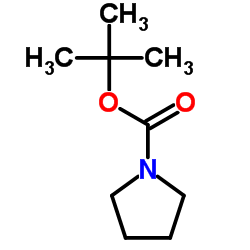 |
2-Methyl-2-propanyl 1-pyrrolidinecarboxylate
CAS:86953-79-9 |
|
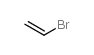 |
bromoethene
CAS:593-60-2 |