| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
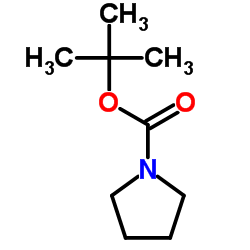 |
1-Boc-四氢吡咯
CAS:86953-79-9 |
|
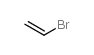 |
溴乙烯
CAS:593-60-2 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
1-Boc-四氢吡咯
CAS:86953-79-9 |
|
 |
溴乙烯
CAS:593-60-2 |