Organic Letters
2010-08-20
Synthesis of the C1-C13 tetraenoate subunit of the chivosazoles.
Ian Paterson, S B Jennifer Kan, Lisa J Gibson
Index: Org. Lett. 12(16) , 3724-7, (2010)
Full Text: HTML
Abstract
Using a combination of asymmetric vinylogous Mukaiyama aldol and Stille cross-coupling reactions, an advanced polyene fragment of the chivosazoles was prepared in a highly stereocontrolled manner. This key C1-C13 pentaene subunit, featuring the conjugated (2E,4Z,6E,8Z)-tetraenoate motif and anti-configured C10 and C11 stereocenters of the chivosazoles, terminates in a (Z)-vinyl bromide for the planned cross-coupling to a northern hemisphere fragment.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
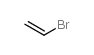 |
bromoethene
CAS:593-60-2 |
C2H3Br |
Related Articles:
More...
|
Reactivity in nucleophilic vinylic substitution (S(N)V):S(N)...
2013-09-06 [J. Org. Chem. 78(17) , 8574-84, (2013)] |
|
Asymmetric synthesis of enantioenriched (+)-elaeokanine A.
2006-07-21 [J. Org. Chem. 71(15) , 5674-8, (2006)] |
|
Formation of enehydrazine intermediates through coupling of ...
2013-01-21 [Angew. Chem. Int. Ed. Engl. 52(4) , 1266-9, (2013)] |
|
Total synthesis of polycavernoside A, a lethal toxin of the ...
2005-07-08 [J. Org. Chem. 70(14) , 5449-60, (2005)] |
|
New one-pot synthesis of (E)-beta-aryl vinyl halides from st...
2009-08-06 [Org. Lett. 11(15) , 3390-3, (2009)] |