| Structure | Name/CAS No. | Articles |
|---|---|---|
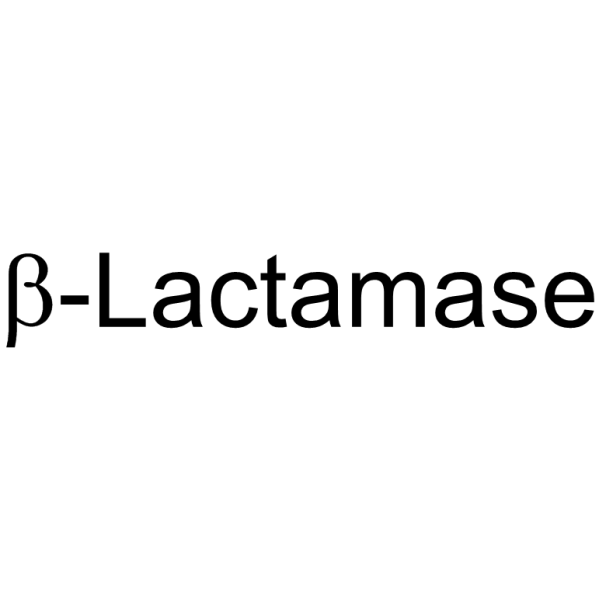 |
β-LactaMase
CAS:9073-60-3 |
|
 |
2-Thiopheneacetyl chloride
CAS:39098-97-0 |