Looking for the new preparations for antibacterial therapy. II. Clinical trials; new beta-lactam antibiotics and beta-lactamase inhibitors.
Izabela Karpiuk, Stefan Tyski
Index: Przegl. Epidemiol. 67(1) , 51-6, 135-40, (2013)
Full Text: HTML
Abstract
To obtain a status of a medicinal product, a compound possessing potential antimicrobial activity and displaying no cytotoxicity, must undergo three phases of clinical trials to prove its therapeutic efficacy, safety and quality. Properties of the compound should be based on the results of studies meeting specific criteria. Studies should be: randomized, double-blind, involving sufficient number of volunteers, concerning the infections localized in strictly defined area and caused by identified microorganisms. After the medicinal product is authorized to be on the market, clinical trials of the fourth phase are carried out to detect adverse effects, overdose symptoms, interactions of the new drug with other medicinal products and to establish characteristic of activity among groups such as children, elderly, women in pregnancy and patients suffering from other diseases, but only if the benefits of receiving treatment outweigh the risks. This article is a second part of the series associated with searching for new antibacterial agents and it relates to performance of clinical trials and the new compounds belonging to the class of beta-lactams. Among the 9 presented compounds, candidates to become medicinal products, two belong to the cephalosporins (CXA-101, S-649266), one to carbapenems (razupenem), three to monobactams (BAL30072, BAL30376, MC-1) and three to beta-lactamase inhibitors (NXL-104, MK-7655, ME1071).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
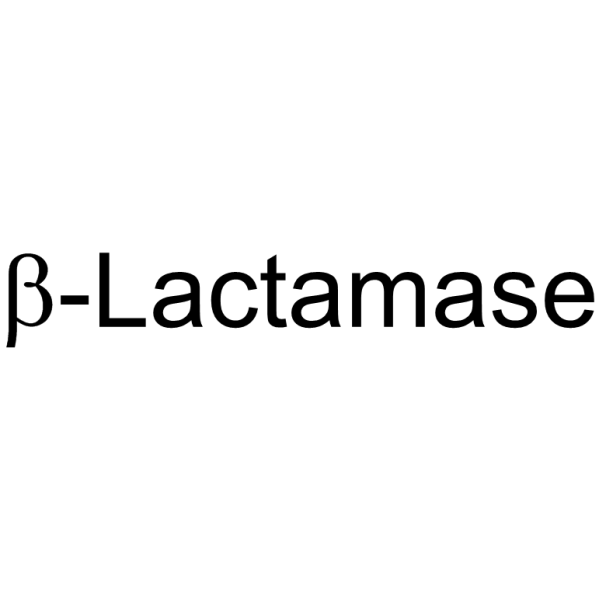 |
β-LactaMase
CAS:9073-60-3 |
|
A Novel Microbisporicin Producer Identified by Early Derepli...
2015-01-01 [Biomed Res. Int. 2015 , 419383, (2015)] |
|
Identification of peptide inhibitors of penicillinase using ...
2016-02-01 [Anal. Biochem. 494 , 4-9, (2015)] |
|
Outbreak of PER-1 and diversity of β-lactamases among ceftaz...
2014-03-01 [J. Med. Microbiol. 63(Pt 3) , 386-92, (2014)] |
|
Comparative in-vitro activity of cefoperazone-tazobactam and...
2012-11-01 [J. Assoc. Physicians India 60 , 22-4, (2012)] |
|
[Fitness costs of blaNDM-1 bearing plasmid pNDM-BJ01 in Acin...
2013-01-04 [Wei Sheng Wu Xue Bao 53(1) , 99-104, (2013)] |