| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
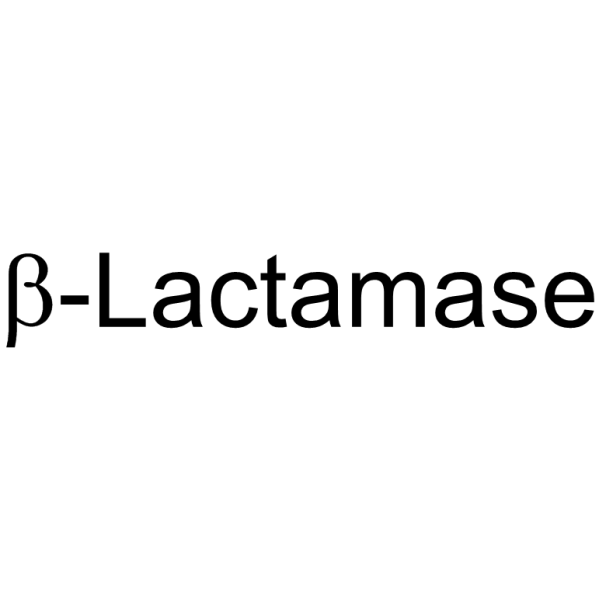 |
β-内酰胺酶
CAS:9073-60-3 |
|
 |
2-噻吩乙酰氯
CAS:39098-97-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
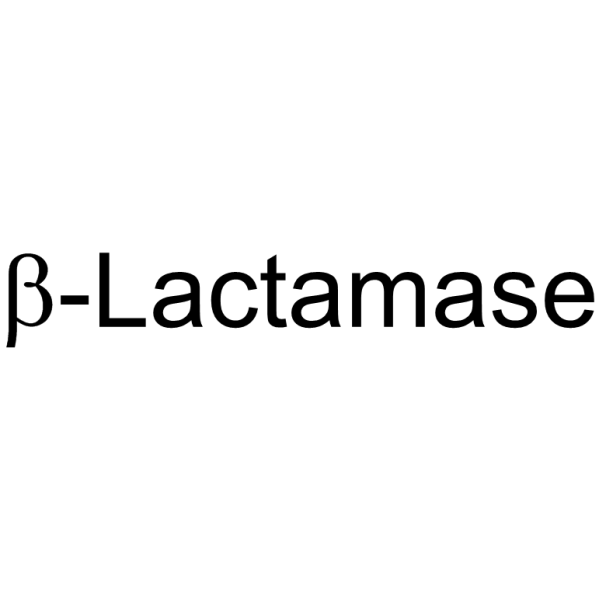 |
β-内酰胺酶
CAS:9073-60-3 |
|
 |
2-噻吩乙酰氯
CAS:39098-97-0 |