| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Colchicine
CAS:64-86-8 |
|
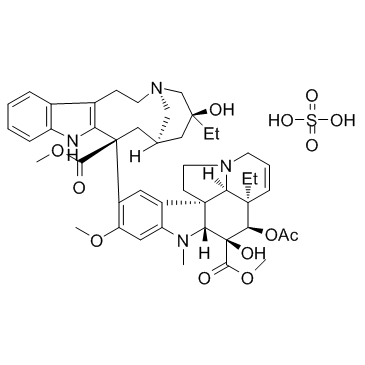 |
Vinblastine Sulfate
CAS:143-67-9 |
|
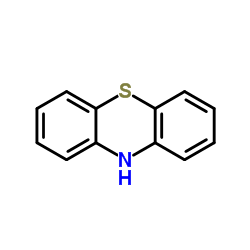 |
Phenothiazine
CAS:92-84-2 |
|
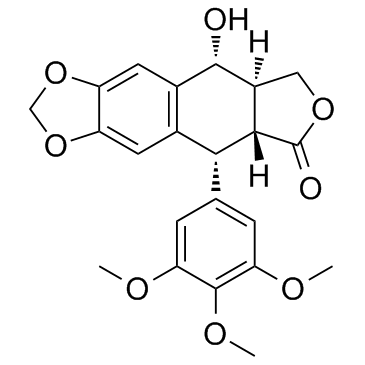 |
Podophyllotoxin
CAS:518-28-5 |
|
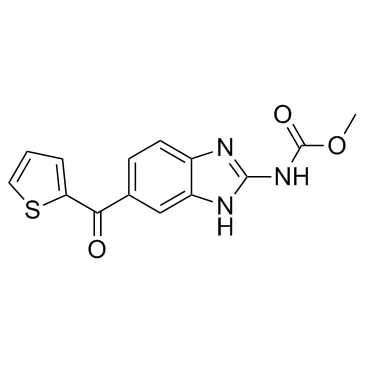 |
Nocodazole
CAS:31430-18-9 |
|
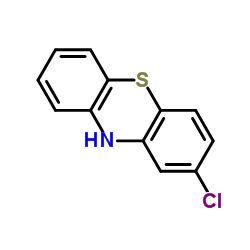 |
2-Chlorophenothiazine
CAS:92-39-7 |