| Structure | Name/CAS No. | Articles |
|---|---|---|
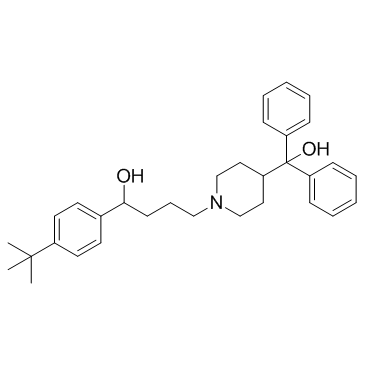 |
Terfenadine
CAS:50679-08-8 |
|
 |
Calmidazolium chloride
CAS:57265-65-3 |
|
 |
Tamoxifen
CAS:10540-29-1 |