Terfenadine
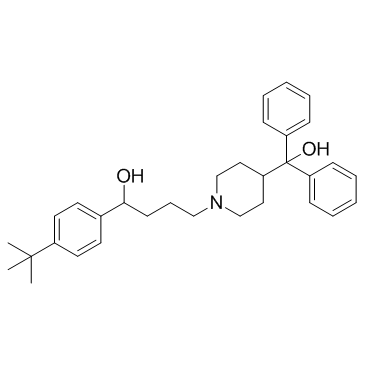
Terfenadine structure
|
Common Name | Terfenadine | ||
|---|---|---|---|---|
| CAS Number | 50679-08-8 | Molecular Weight | 471.673 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 626.8±55.0 °C at 760 mmHg | |
| Molecular Formula | C32H41NO2 | Melting Point | 145-152 °C | |
| MSDS | USA | Flash Point | 306.9±30.2 °C | |
|
Characterization of a highly sensitive and selective novel trapping reagent, stable isotope labeled glutathione ethyl ester, for the detection of reactive metabolites.
J. Pharmacol. Toxicol. Methods 76 , 83-95, (2015) Glutathione (GSH) trapping assays are widely used to predict the post-marketing risk for idiosyncratic drug reactions (IDRs) in the pharmaceutical industry. Although several GSH derivatives have been introduced as trapping reagents for reactive intermediates,... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental drug discovery projects toward safer medicines. In this st... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI types (hepatotoxic side effects) seen in the clinic can be tra... |
|
|
Calculating virtual log P in the alkane/water system (log P(N)(alk)) and its derived parameters deltalog P(N)(oct-alk) and log D(pH)(alk).
J. Med. Chem. 48 , 3269-79, (2005) Growing interest in the use of both the logarithm of the partition coefficient of the neutral species in the alkane/water system (log P(N)(alk)) and the difference between log P(N)(oct) (the logarithm of the partition coefficient of the neutral species in the... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals can make a significant contribution to the identification o... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predictive in vivo, in vitro, and in silico models to identify comp... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain cancer. However, the complete repertoire of signaling pathways ... |
|
|
Metabolomic discovery of novel urinary galabiosylceramide analogs as Fabry disease biomarkers.
J. Am. Soc. Mass Spectrom. 26(3) , 499-510, (2015) Fabry disease is an X-linked, complex, multisystemic lysosomal storage disorder presenting marked phenotypic and genotypic variability among affected male and female patients. Glycosphingolipids, mainly globotriaosylceramide (Gb(3)) isoforms/analogs, globotri... |
|
|
Altered plasma lysophosphatidylcholines and amides in non-obese and non-diabetic subjects with borderline-to-moderate hypertriglyceridemia: a case-control study.
PLoS ONE 10(4) , e0123306, (2015) Hypertriglyceridemia (HTG) is a risk factor for atherosclerotic cardiovascular disease (CVD). We investigated alterations in plasma metabolites associated with borderline-to-moderate HTG (triglycerides (TG) 150-500 mg/dL). Using UPLC-LTQ-Orbitrap mass spectro... |
|
|
Structural requirements for drug inhibition of the liver specific human organic cation transport protein 1.
J. Med. Chem. 51 , 5932-42, (2008) The liver-specific organic cation transport protein (OCT1; SLC22A1) transports several cationic drugs including the antidiabetic drug metformin and the anticancer agents oxaliplatin and imatinib. In this study, we explored the chemical space of registered ora... |