| Structure | Name/CAS No. | Articles |
|---|---|---|
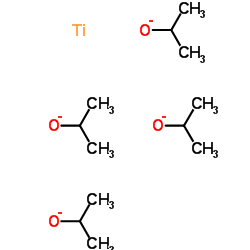 |
Titanium(4+) tetrapropan-2-olate
CAS:546-68-9 |
|
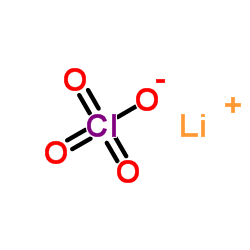 |
Lithium perchlorate
CAS:7791-03-9 |
|
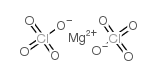 |
Magnesium perchlorate
CAS:10034-81-8 |
|
 |
Lithium perchlorate trihydrate
CAS:13453-78-6 |