| Structure | Name/CAS No. | Articles |
|---|---|---|
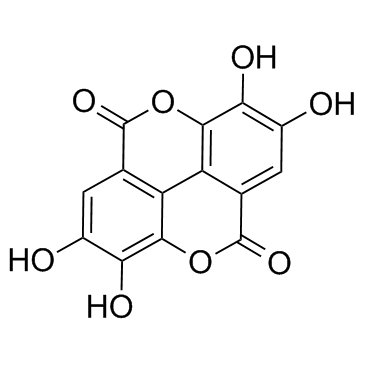 |
Ellagic acid
CAS:476-66-4 |
|
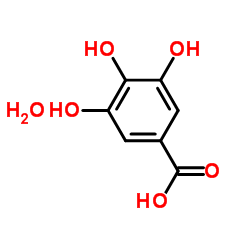 |
Gallic acid hydrate
CAS:5995-86-8 |
|
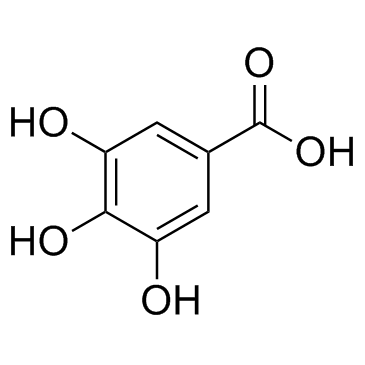 |
Gallic acid
CAS:149-91-7 |
|
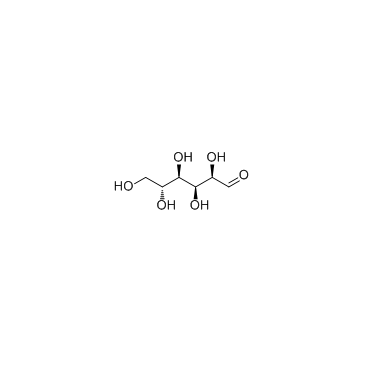 |
D-(+)-Glucose
CAS:50-99-7 |
|
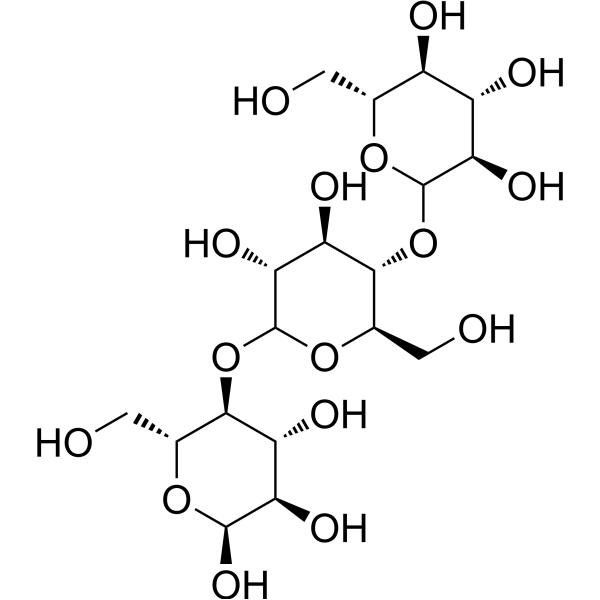 |
α-D-Glucopyranose
CAS:9004-53-9 |
|
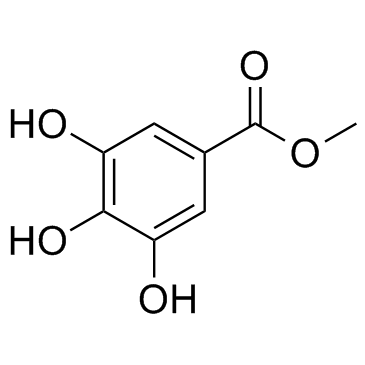 |
Methyl gallate
CAS:99-24-1 |
|
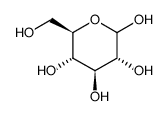 |
dextrose
CAS:492-62-6 |