| Structure | Name/CAS No. | Articles |
|---|---|---|
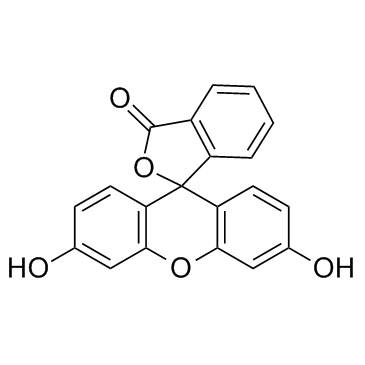 |
Fluorescein
CAS:2321-07-5 |
|
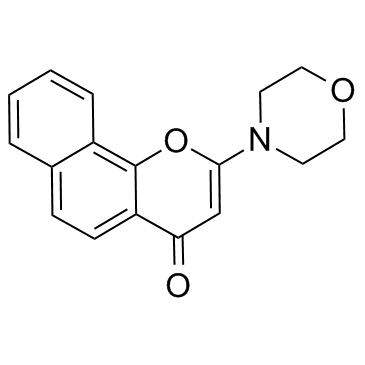 |
NU7026
CAS:154447-35-5 |
|
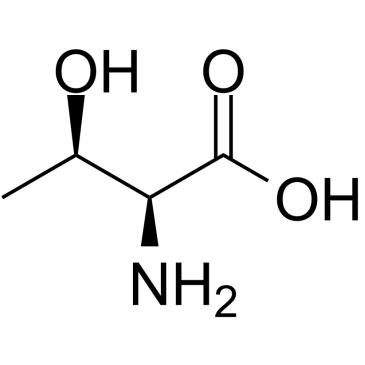 |
L-Threonine
CAS:72-19-5 |
|
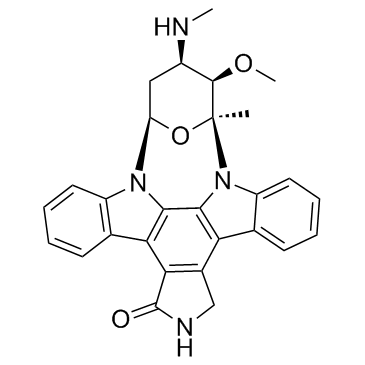 |
Staurosporine
CAS:62996-74-1 |
|
 |
4',6-Diamidino-2-phenylindole dihydrochloride
CAS:28718-90-3 |