| Structure | Name/CAS No. | Articles |
|---|---|---|
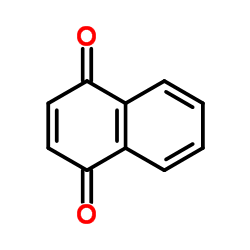 |
1,4-naphthoquinone
CAS:130-15-4 |
|
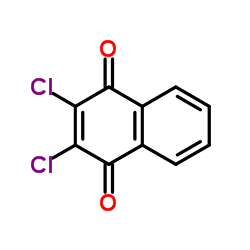 |
2,3-Dichlor-1,4-naphthochinone
CAS:117-80-6 |
|
 |
Vitamin K1
CAS:84-80-0 |
|
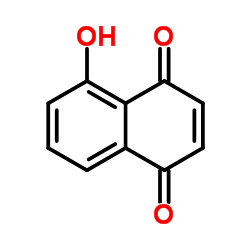 |
Juglone
CAS:481-39-0 |
|
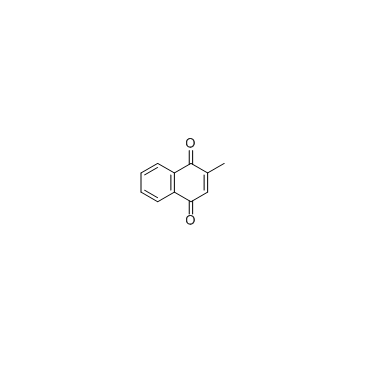 |
Menadione
CAS:58-27-5 |
|
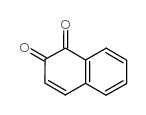 |
1,2-NAPHTHOQUINONE
CAS:524-42-5 |
|
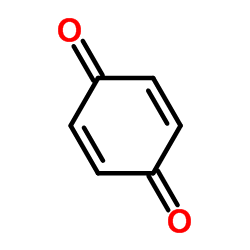 |
1,4-Benzoquinone
CAS:106-51-4 |
|
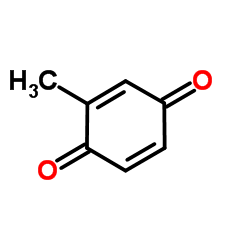 |
2-Methylcyclohexa-2,5-diene-1,4-dione
CAS:553-97-9 |