| Structure | Name/CAS No. | Articles |
|---|---|---|
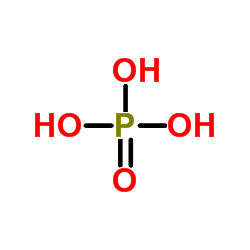 |
Phosphoric acid
CAS:7664-38-2 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
 |
sodium dihydrogenphosphate
CAS:7558-80-7 |
|
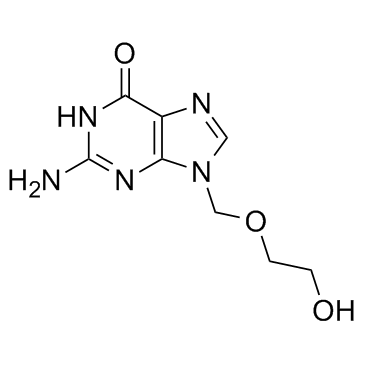 |
Acyclovir
CAS:59277-89-3 |
|
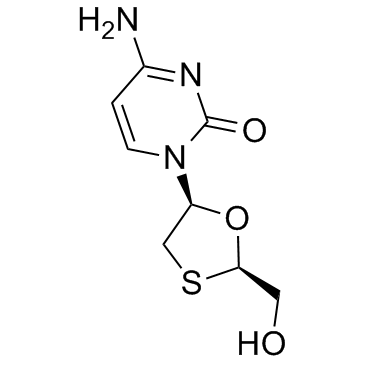 |
Lamivudine
CAS:134678-17-4 |