| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Phenol
CAS:108-95-2 |
|
 |
Piperidine
CAS:110-89-4 |
|
 |
Aniline
CAS:62-53-3 |
|
 |
DIEA
CAS:7087-68-5 |
|
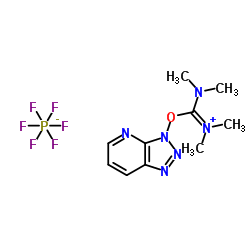 |
HATU
CAS:148893-10-1 |
|
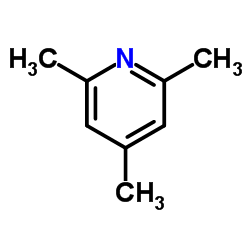 |
2,4,6-Trimethylpyridine
CAS:108-75-8 |