| Structure | Name/CAS No. | Articles |
|---|---|---|
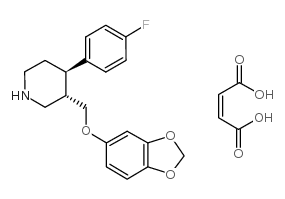 |
Paroxetine maleate
CAS:64006-44-6 |
|
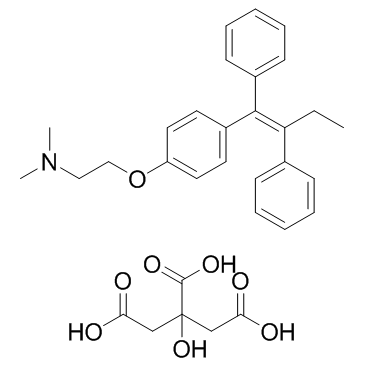 |
Tamoxifen citrate
CAS:54965-24-1 |
|
 |
Tamoxifen
CAS:10540-29-1 |
|
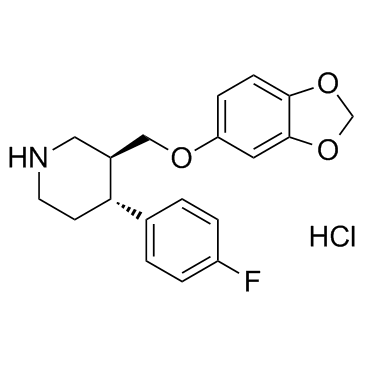 |
Paroxetine HCl
CAS:78246-49-8 |