| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
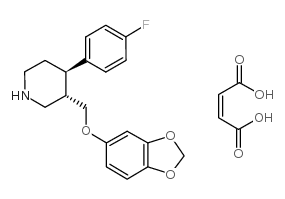 |
帕罗西汀 马来酸盐
CAS:64006-44-6 |
|
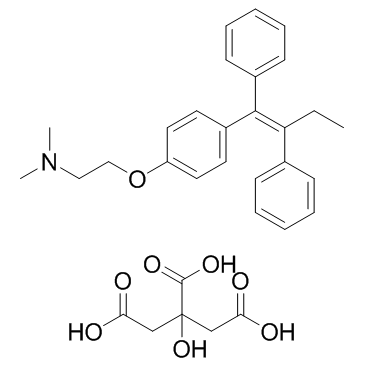 |
枸橼酸他莫昔芬
CAS:54965-24-1 |
|
 |
他莫昔芬
CAS:10540-29-1 |
|
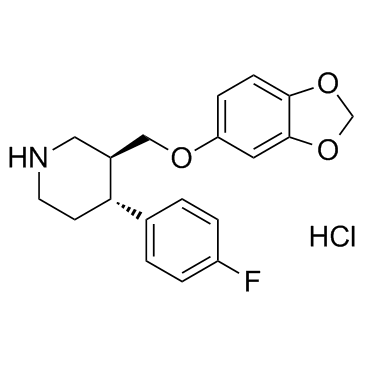 |
盐酸帕罗西汀
CAS:78246-49-8 |