| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
酮康唑
CAS:65277-42-1 |
|
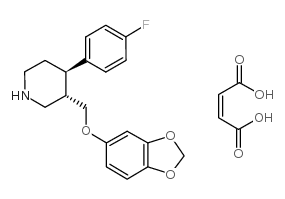 |
帕罗西汀 马来酸盐
CAS:64006-44-6 |
|
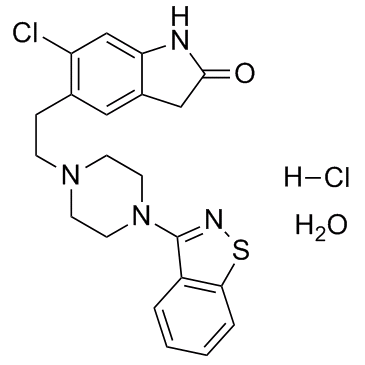 |
盐酸齐拉西酮
CAS:138982-67-9 |
|
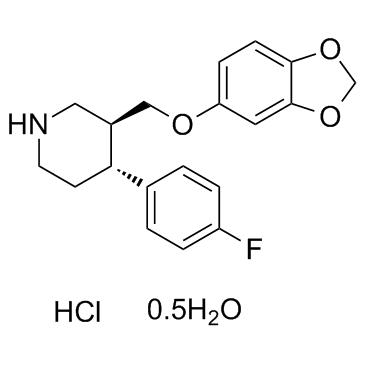 |
盐酸帕罗西汀半水合物
CAS:110429-35-1 |
|
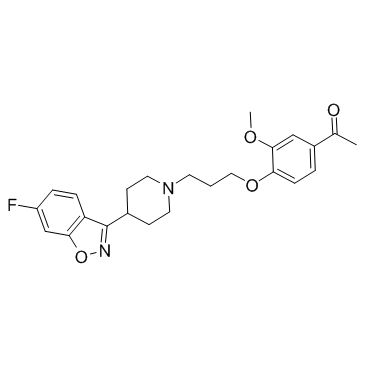 |
伊潘立酮
CAS:133454-47-4 |
|
 |
齐拉西酮
CAS:146939-27-7 |