| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethanol
CAS:64-17-5 |
|
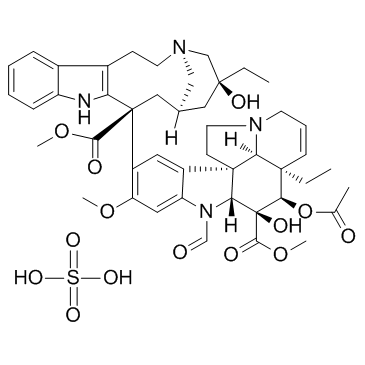 |
Vincristine Sulfate
CAS:2068-78-2 |
|
 |
Isopropanol
CAS:67-63-0 |
|
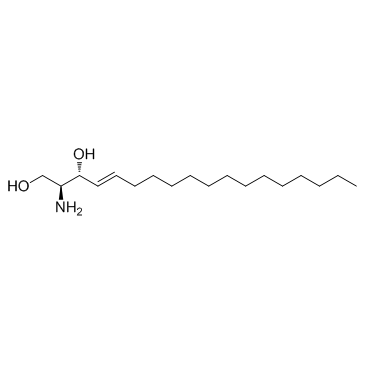 |
D-erythro-Sphingosine
CAS:123-78-4 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
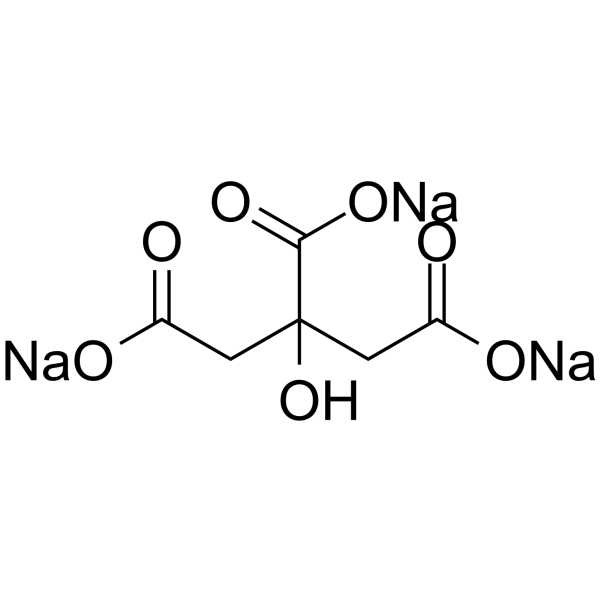 |
Sodium citrate
CAS:68-04-2 |
|
 |
ethyl acetate
CAS:141-78-6 |
|
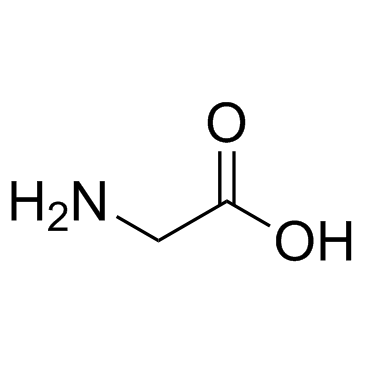 |
Glycine
CAS:56-40-6 |
|
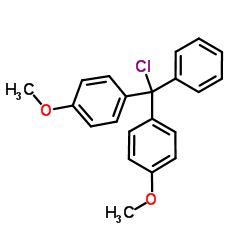 |
4,4'-Dimethoxytrityl chloride
CAS:40615-36-9 |
|
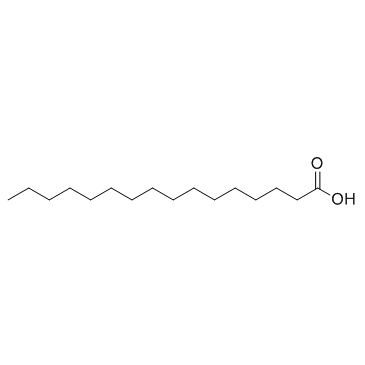 |
Palmitic acid
CAS:57-10-3 |