| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethanol
CAS:64-17-5 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
 |
Formic Acid
CAS:64-18-6 |
|
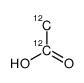 |
acetic acid
CAS:1173022-32-6 |
|
 |
acetic acid
CAS:64-19-7 |
|
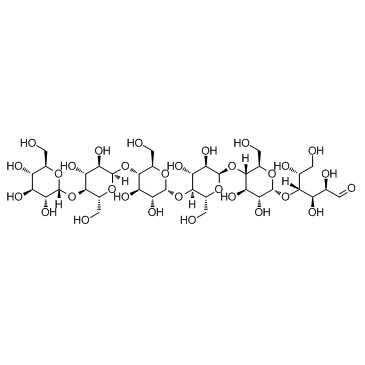 |
Maltohexaose
CAS:34620-77-4 |
|
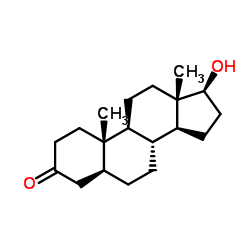 |
Stanolone
CAS:521-18-6 |
|
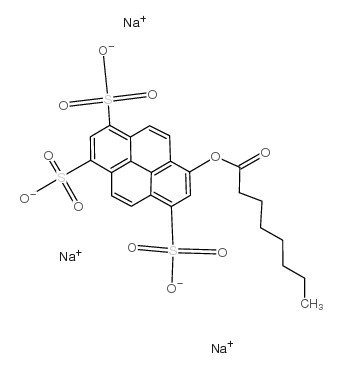 |
8-Octanoyloxypyrene-1,3,6-trisulfonic acid trisodium salt
CAS:115787-84-3 |