| Structure | Name/CAS No. | Articles |
|---|---|---|
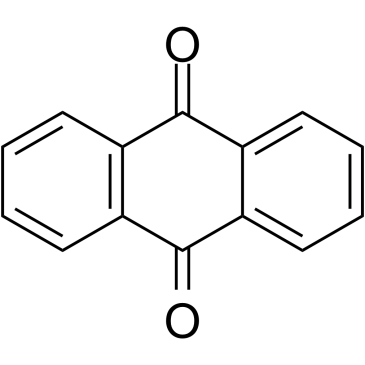 |
Anthraquinone
CAS:84-65-1 |
|
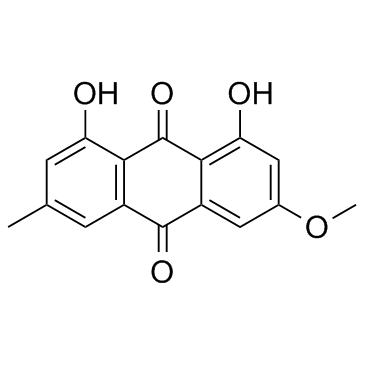 |
Physcion
CAS:521-61-9 |
|
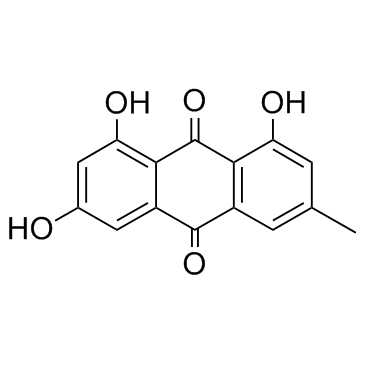 |
Emodin
CAS:518-82-1 |
|
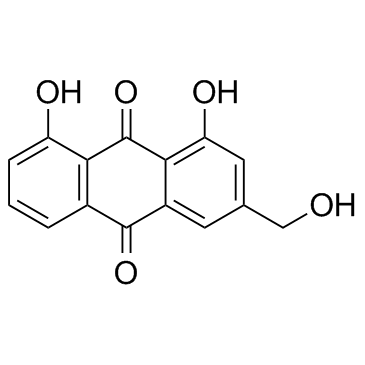 |
Aloeemodin
CAS:481-72-1 |