| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Isopropanol
CAS:67-63-0 |
|
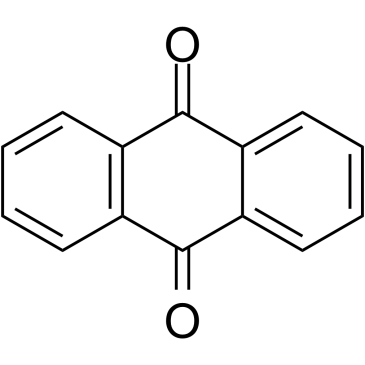 |
Anthraquinone
CAS:84-65-1 |
|
 |
Ethanol
CAS:64-17-5 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
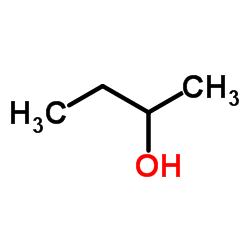 |
(±)-2-Butanol
CAS:78-92-2 |
|
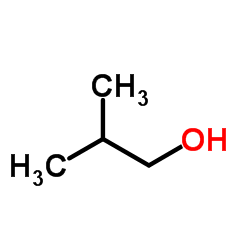 |
isobutanol
CAS:78-83-1 |
|
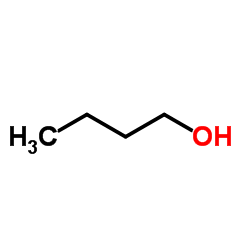 |
Butanol
CAS:71-36-3 |
|
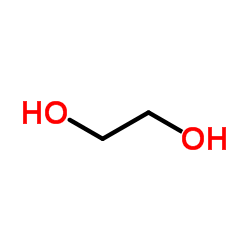 |
Ethylene glycol
CAS:107-21-1 |