| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
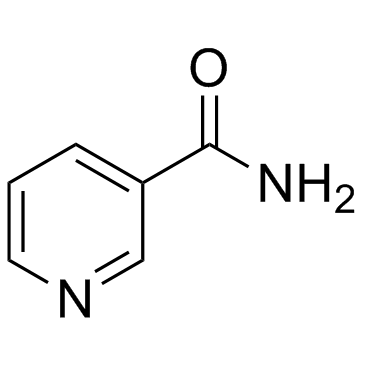 |
Nicotinamide
CAS:98-92-0 |
|
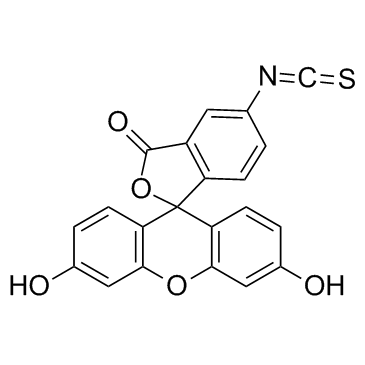 |
Fluorescein isothiocyanate
CAS:3326-32-7 |
|
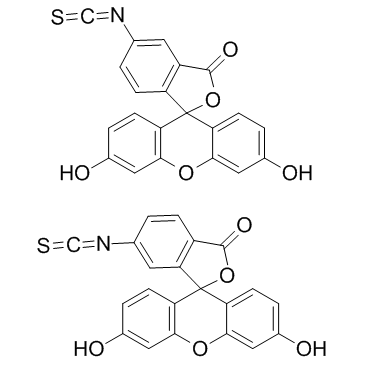 |
fluorescein 5-isothiocyanate
CAS:27072-45-3 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
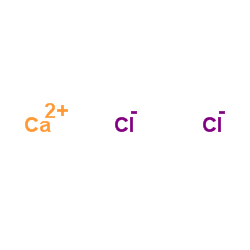 |
Calcium chloride
CAS:10043-52-4 |
|
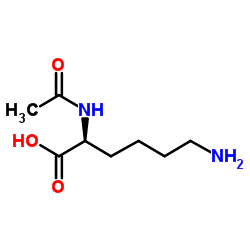 |
AC-Lys-OH
CAS:1946-82-3 |
|
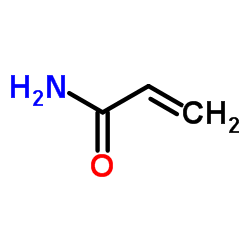 |
Acrylamide Crystals
CAS:79-06-1 |
|
 |
magnesium sulfate
CAS:7487-88-9 |