| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
Acetone
CAS:67-64-1 |
|
 |
Isopropanol
CAS:67-63-0 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
 |
dichloroethane
CAS:107-06-2 |
|
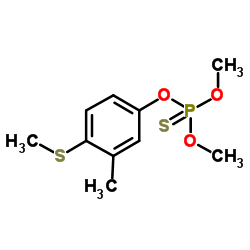 |
mpp
CAS:55-38-9 |
|
 |
N,N-Dimethylformamide
CAS:68-12-2 |
|
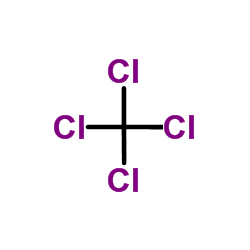 |
Carbon tetrachloride
CAS:56-23-5 |