| Structure | Name/CAS No. | Articles |
|---|---|---|
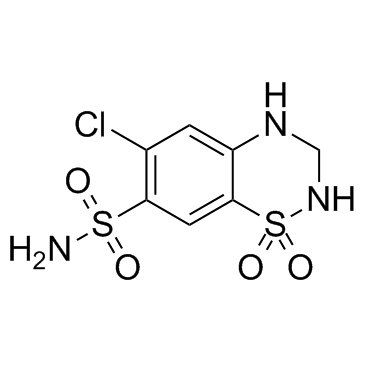 |
Hydrochlorothiazide
CAS:58-93-5 |
|
 |
Piroxicam
CAS:36322-90-4 |
|
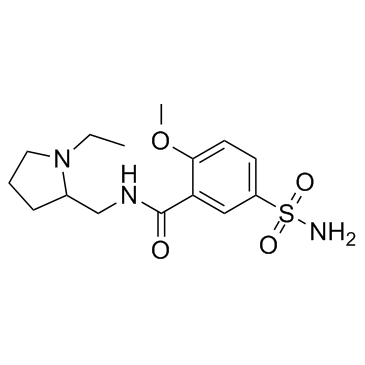 |
Sulpiride
CAS:15676-16-1 |
|
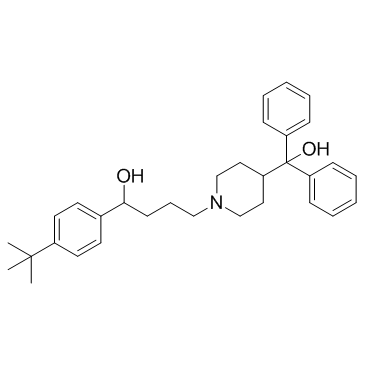 |
Terfenadine
CAS:50679-08-8 |
|
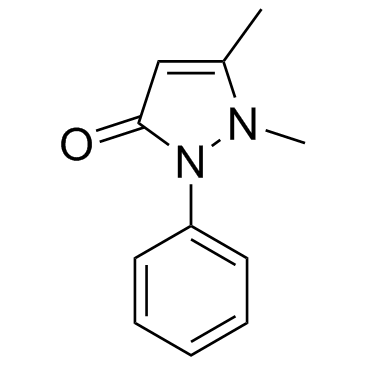 |
Antipyrine
CAS:60-80-0 |
|
 |
Furosemide
CAS:54-31-9 |
|
 |
Caffeine
CAS:58-08-2 |
|
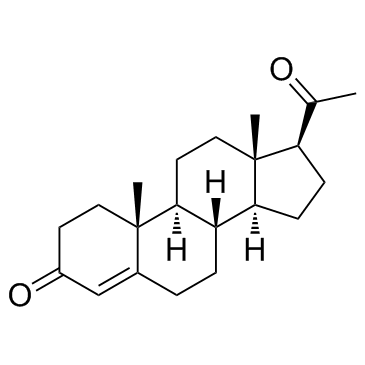 |
Progesterone
CAS:57-83-0 |
|
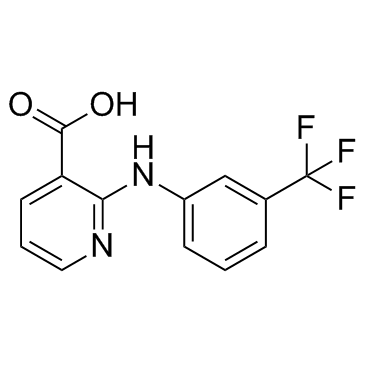 |
Niflumic acid
CAS:4394-00-7 |
|
 |
Hydrocortisone
CAS:50-23-7 |