| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetone
CAS:67-64-1 |
|
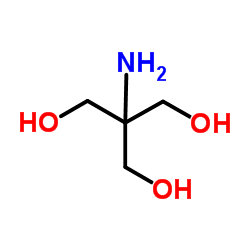 |
Trometamol
CAS:77-86-1 |
|
 |
Diethyl ether
CAS:60-29-7 |
|
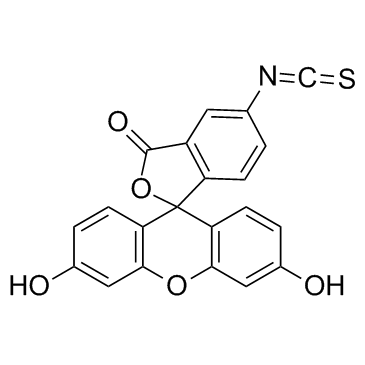 |
Fluorescein isothiocyanate
CAS:3326-32-7 |
|
 |
Methanol
CAS:67-56-1 |
|
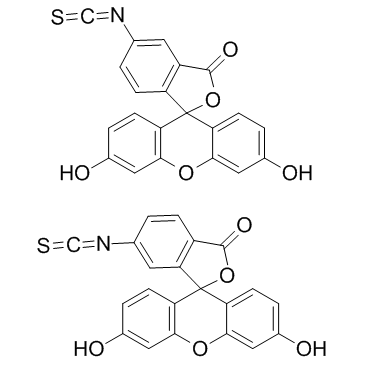 |
fluorescein 5-isothiocyanate
CAS:27072-45-3 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
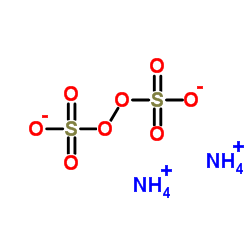 |
ammonium persulfate
CAS:7727-54-0 |
|
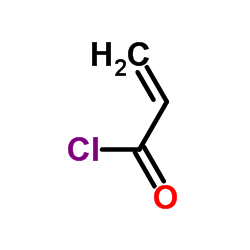 |
Acrylyl chloride
CAS:814-68-6 |
|
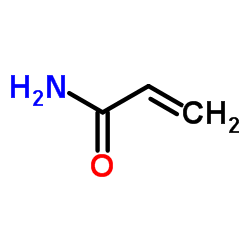 |
Acrylamide Crystals
CAS:79-06-1 |