| Structure | Name/CAS No. | Articles |
|---|---|---|
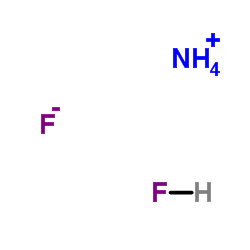 |
Ammonium bifluoride
CAS:1341-49-7 |
|
 |
HEPES
CAS:7365-45-9 |
|
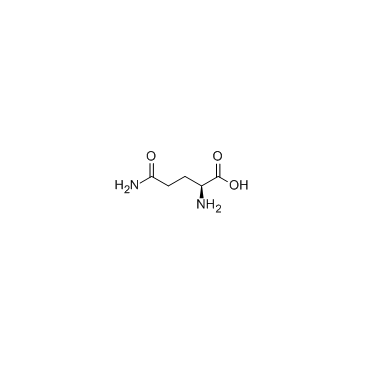 |
L-Glutamine
CAS:56-85-9 |
|
 |
dimercaprol
CAS:59-52-9 |
|
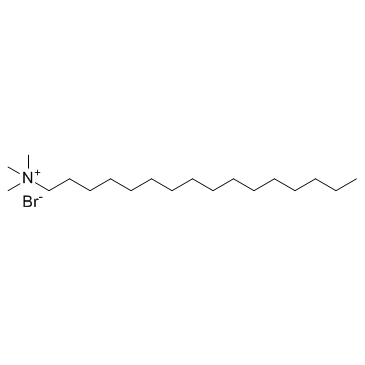 |
Hexadecyl trimethyl ammonium bromide
CAS:57-09-0 |
|
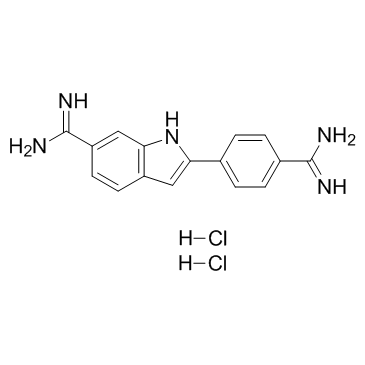 |
4',6-Diamidino-2-phenylindole dihydrochloride
CAS:28718-90-3 |
|
 |
Ammonium Chloride
CAS:12125-02-9 |
|
 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |
|
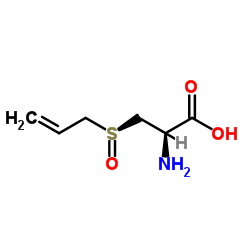 |
(±)-Alliin
CAS:17795-26-5 |
|
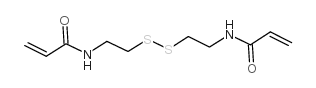 |
N,N'-Bis(Acryloyl)Cystamine
CAS:60984-57-8 |