| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
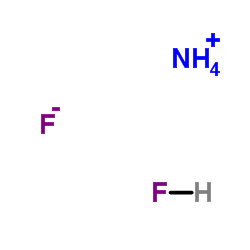 |
氟化氢铵
CAS:1341-49-7 |
|
 |
4-羟乙基哌嗪乙磺酸
CAS:7365-45-9 |
|
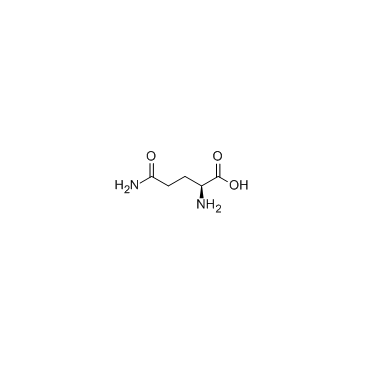 |
L-谷氨酰胺
CAS:56-85-9 |
|
 |
2,3-二巯基丙醇
CAS:59-52-9 |
|
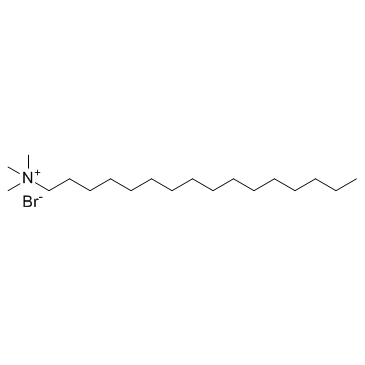 |
十六烷基三甲基溴化铵
CAS:57-09-0 |
|
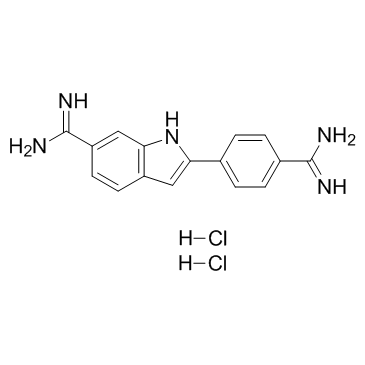 |
4',6-二脒基-2-苯基吲哚二盐酸盐
CAS:28718-90-3 |
|
 |
氯化铵
CAS:12125-02-9 |
|
 |
乙二胺四乙酸
CAS:60-00-4 |
|
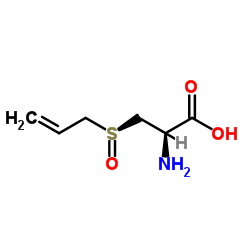 |
S-烯丙基-L-半胱氨酸
CAS:17795-26-5 |
|
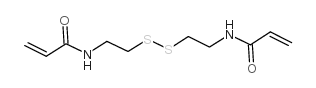 |
N,N'-双(丙稀酰)胱胺
CAS:60984-57-8 |