| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
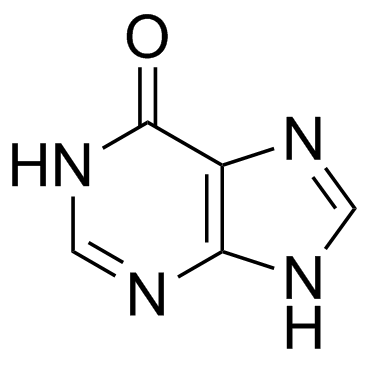 |
Hypoxanthine
CAS:68-94-0 |
|
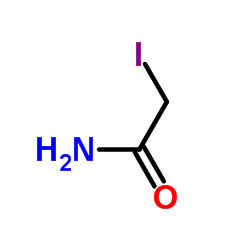 |
Iodoacetamide
CAS:144-48-9 |
|
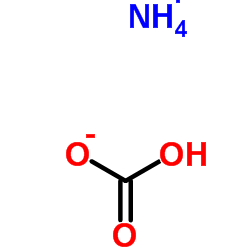 |
Ammonium Bicarbonate
CAS:1066-33-7 |
|
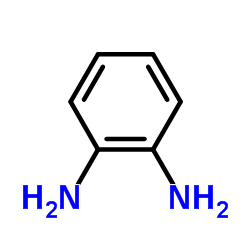 |
o-Phenylenediamine
CAS:95-54-5 |
|
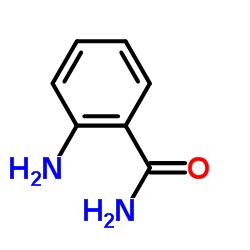 |
Anthranilamide
CAS:88-68-6 |
|
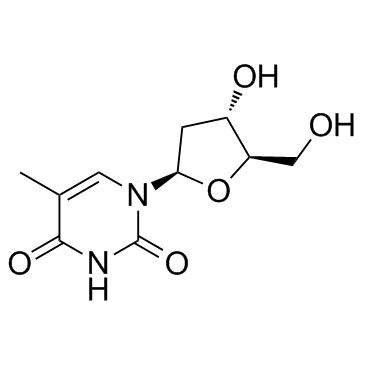 |
Thymidine
CAS:50-89-5 |
|
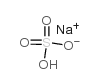 |
Sodium bisulfate
CAS:7681-38-1 |