| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Methanol
CAS:67-56-1 |
|
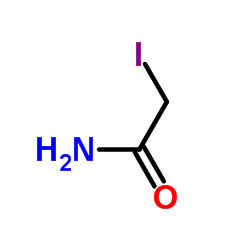 |
Iodoacetamide
CAS:144-48-9 |
|
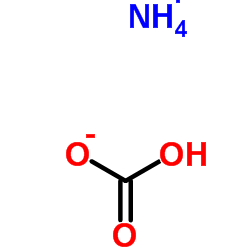 |
Ammonium Bicarbonate
CAS:1066-33-7 |
|
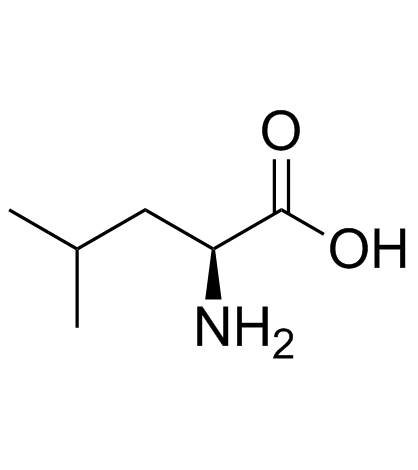 |
L-leucine
CAS:61-90-5 |
|
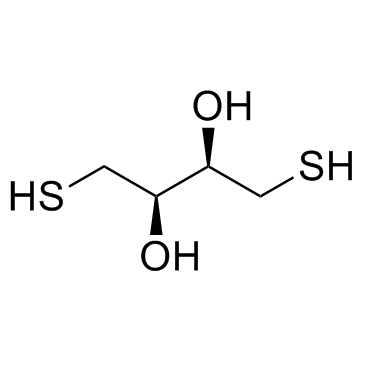 |
DL-Dithiothreitol
CAS:3483-12-3 |
|
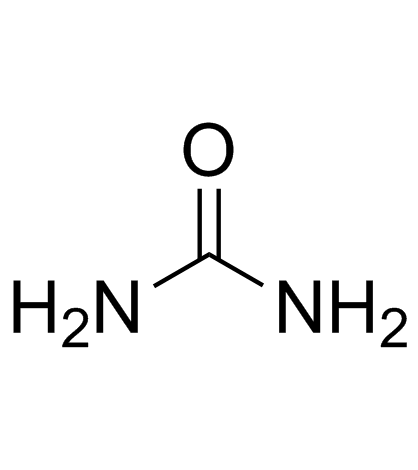 |
Urea
CAS:57-13-6 |
|
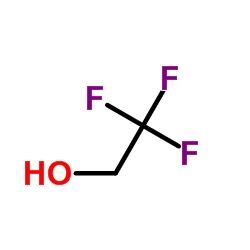 |
2,2,2-Trifluoroethanol
CAS:75-89-8 |
|
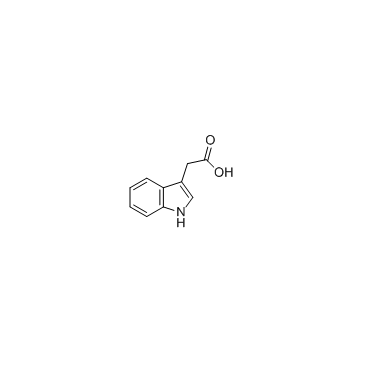 |
3-Indoleacetic acid
CAS:87-51-4 |
|
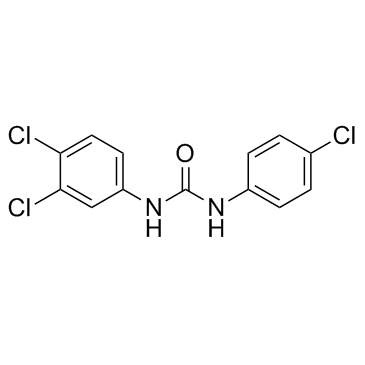 |
Triclocarban
CAS:101-20-2 |