| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Propofol
CAS:2078-54-8 |
|
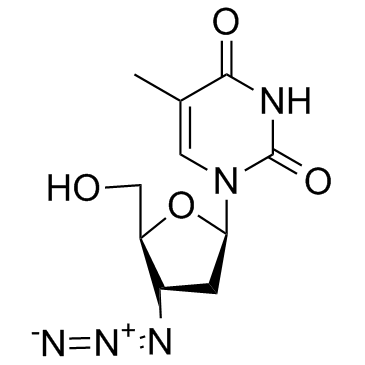 |
Zidovudine
CAS:30516-87-1 |
|
 |
1-Hydroxypyrene
CAS:5315-79-7 |
|
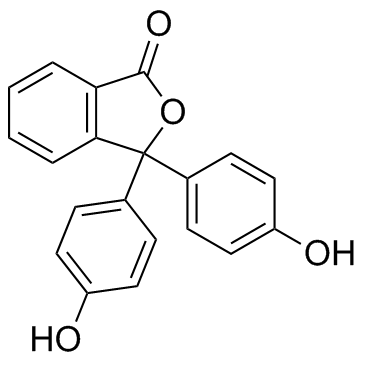 |
Phenolphthalein
CAS:77-09-8 |
|
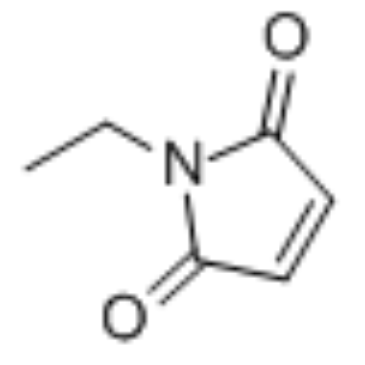 |
N-ethylmaleimide
CAS:128-53-0 |
|
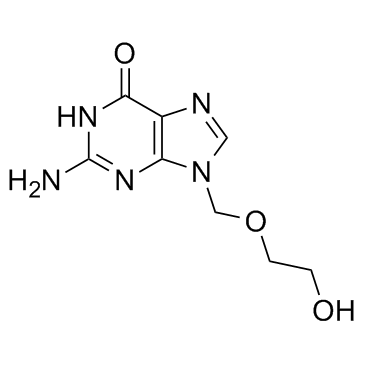 |
Acyclovir
CAS:59277-89-3 |
|
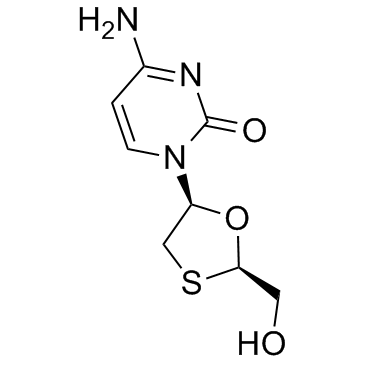 |
Lamivudine
CAS:134678-17-4 |
|
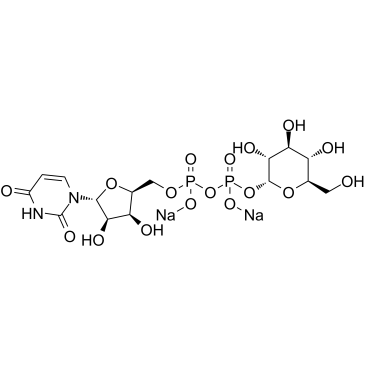 |
Uridine 5′-diphosphoglucose disodium salt
CAS:28053-08-9 |