| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethidium bromide
CAS:1239-45-8 |
|
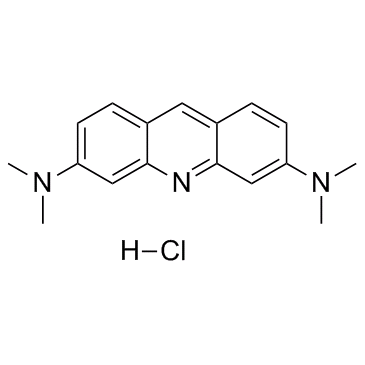 |
Acridine Orange hydrochloride
CAS:65-61-2 |
|
 |
Salicylic acid
CAS:69-72-7 |
|
 |
Shikimic acid
CAS:138-59-0 |
|
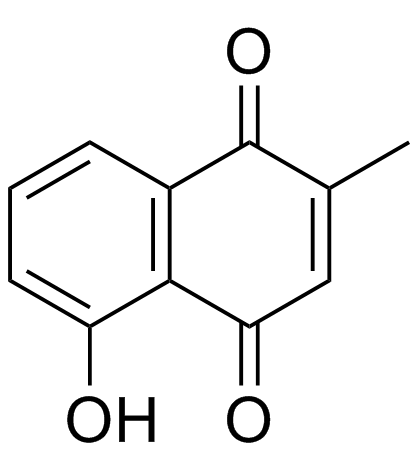 |
Plumbagin
CAS:481-42-5 |
|
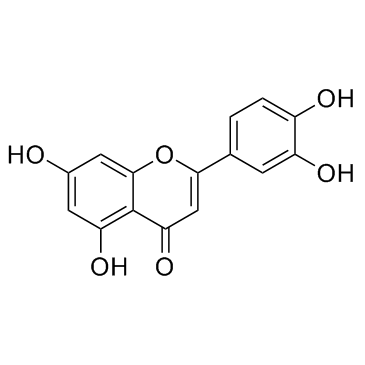 |
Luteolin
CAS:491-70-3 |
|
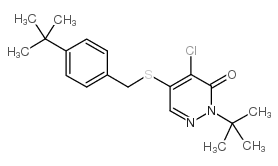 |
Pyridaben
CAS:96489-71-3 |
|
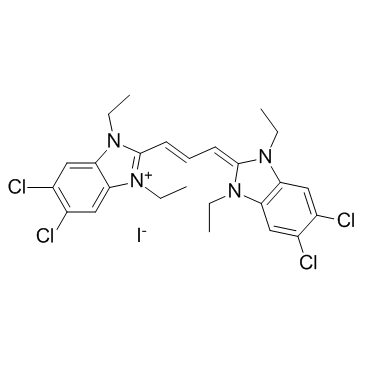 |
JC-1
CAS:3520-43-2 |
|
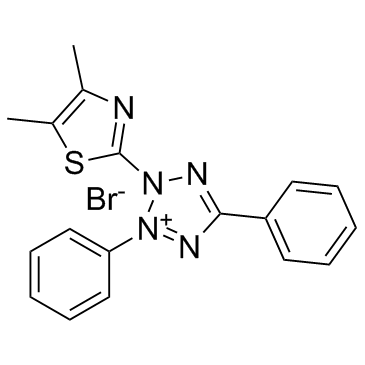 |
Thiazolyl Blue
CAS:298-93-1 |