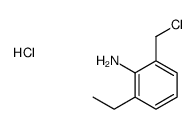
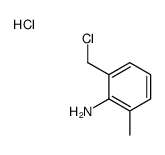
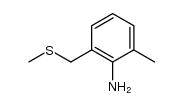
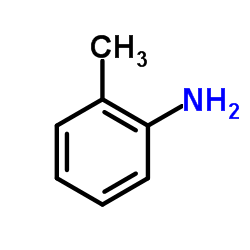
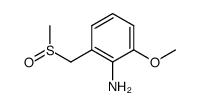
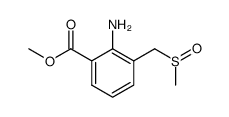
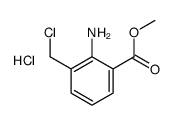
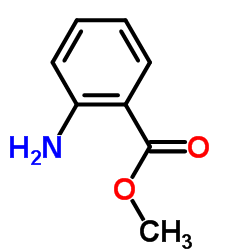
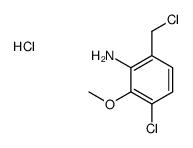
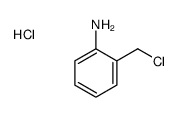
|
~97% |
|
~60% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~87% |
|
~% |
|
~% |
|
~88% |
|
~% |
|
~58% |
|
~86% |
|
~% |
|
~85% |
|
~% |
|
~% |
|
~85% |
|
~% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~96% |
|
~% |
|
~% |
|
~69% |
|
~90% |
|
~% |
![[2-Amino-3-(trifluoromethyl)benzyl]trimethylammonoium chloride Structure](https://image.chemsrc.com/caspic/321/88301-97-7.png)
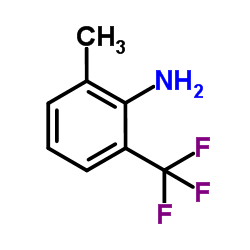
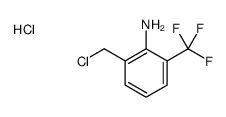

![N-[2-(chloromethyl)-6-(trifluoromethyl)phenyl]acetamide Structure](https://image.chemsrc.com/caspic/218/88301-82-0.png)


![2'-[(Methylthio)methyl]acetanilide Structure](https://image.chemsrc.com/caspic/025/65134-90-9.png)



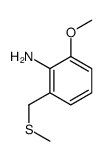
![2-Ethyl-6-[(methylsulfinyl)methyl]benzenamine Structure](https://www.chemsrc.com/extcaspic/446/92643-48-6.png)