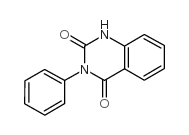
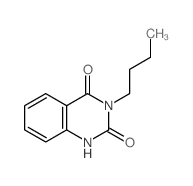
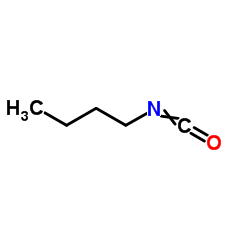
|
~97% |
|
~% |
|
~% |
|
~99% |
|
~% |
|
~94% |
|
~63% |
|
~% |
|
~% |
|
~89% |
|
~% |
|
~98% |
|
~88% |
|
~% |
|
~% |
|
~% |
|
~10% |
|
~96% |
|
~% |
![methyl 2-[(4-chlorophenyl)carbamoylamino]benzoate Structure](https://image.chemsrc.com/caspic/402/19959-41-2.png)
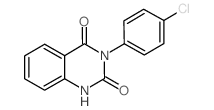
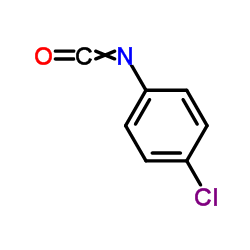
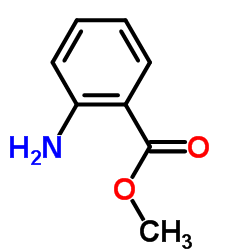
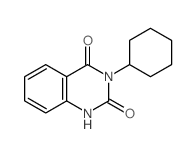
![9-(cyclohexylamino)-8-oxa-10-azabicyclo[4.4.0]deca-1,3,5,9-tetraen-7-one Structure](https://image.chemsrc.com/caspic/466/81904-99-6.png)
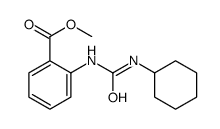
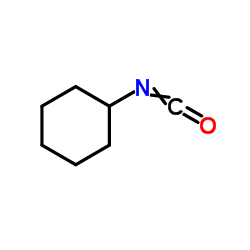
![Benzoic acid,2-[(aminocarbonyl)amino]-, methyl ester Structure](https://image.chemsrc.com/caspic/355/2242-77-5.png)
![2-AMINO-4H-BENZO[D][1,3]OXAZIN-4-ONE Structure](https://image.chemsrc.com/caspic/168/15607-11-1.png)
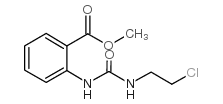
![9-(2-chloroethylamino)-8-oxa-10-azabicyclo[4.4.0]deca-1,3,5,9-tetraen-7-one Structure](https://image.chemsrc.com/caspic/075/77093-93-7.png)

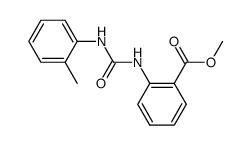
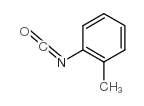
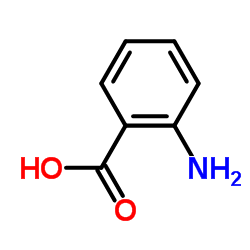
![Benzoic acid,2-[[(phenylamino)carbonyl]amino]-, methyl ester Structure](https://image.chemsrc.com/caspic/371/2321-50-8.png)