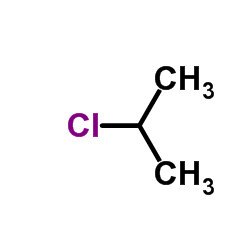
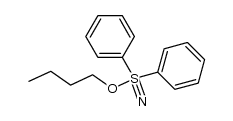
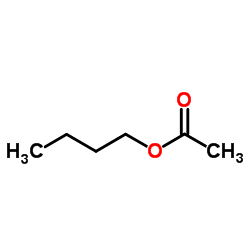
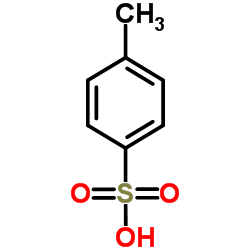
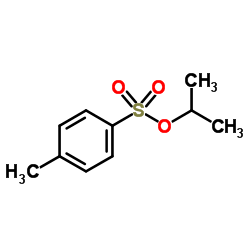
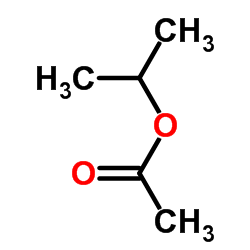
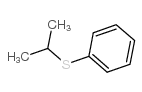
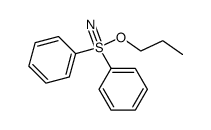
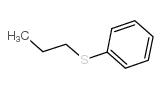
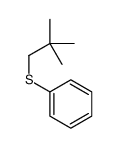
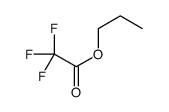
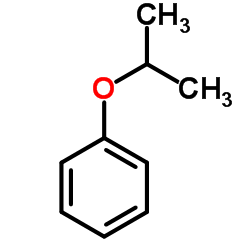
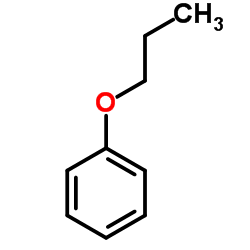
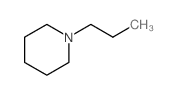
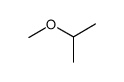
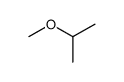
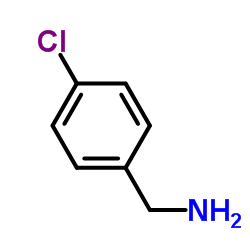
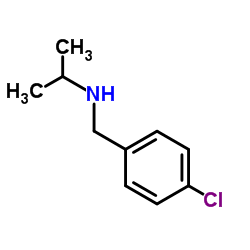
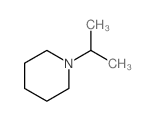
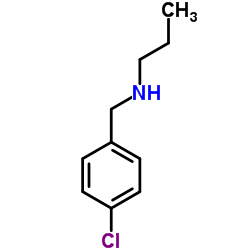
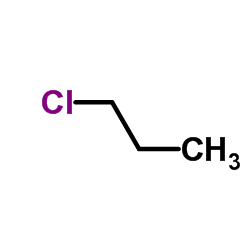
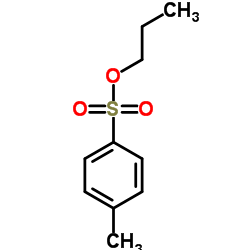
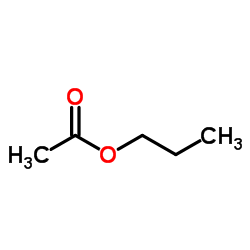
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~86% |
|
~96% |
|
~0% |
|
~0% |
|
~0% |
|
~48% |
|
~95% |
|
~96% |
|
~98% |
|
~97% |
|
~96% |
|
~90% |
|
~98% |
|
~92% |
|
~99% |
|
~97% |
|
~19% |
|
~89% |
|
~96% |
|
~67% |
|
~90% |
|
~96% |