Pivaldehyde

Pivaldehyde structure
|
Common Name | Pivaldehyde | ||
|---|---|---|---|---|
| CAS Number | 630-19-3 | Molecular Weight | 86.132 | |
| Density | 0.8±0.1 g/cm3 | Boiling Point | 77.5±0.0 °C at 760 mmHg | |
| Molecular Formula | C5H10O | Melting Point | 6 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | -13.2±7.8 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
| Name | Trimethylacetaldehyde |
|---|---|
| Synonym | More Synonyms |
| Density | 0.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 77.5±0.0 °C at 760 mmHg |
| Melting Point | 6 °C(lit.) |
| Molecular Formula | C5H10O |
| Molecular Weight | 86.132 |
| Flash Point | -13.2±7.8 °C |
| Exact Mass | 86.073166 |
| PSA | 17.07000 |
| LogP | 1.07 |
| Vapour Pressure | 96.5±0.1 mmHg at 25°C |
| Index of Refraction | 1.384 |
| InChIKey | FJJYHTVHBVXEEQ-UHFFFAOYSA-N |
| SMILES | CC(C)(C)C=O |
| Storage condition | 2-8°C |
| Water Solubility | Negligible |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H315-H335 |
| Precautionary Statements | P210-P261 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | F:Flammable |
| Risk Phrases | R11 |
| Safety Phrases | S16-S23 |
| RIDADR | UN 1989 3/PG 2 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 3 |
| HS Code | 2912190090 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |
| HS Code | 2912190090 |
|---|---|
| Summary | 2912190090 acyclic aldehydes without other oxygen function。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Tetrahedron Asymmetry 17 , 2659, (2006)
|
|
|
Double stereodifferentiation in the catalytic asymmetric aziridination of imines prepared from α-chiral amines.
Chemistry 18(17) , 5302-13, (2012) The catalytic asymmetric aziridination of imines and diazo compounds (AZ reaction) mediated by boroxinate catalysts derived from the VANOL and VAPOL ligands was investigated with chiral imines derived... |
|
|
Diastereomeric Reissert compounds of isoquinoline and 6,7-dimethoxy-3,4-dihydroisoquinoline in stereoselective synthesis.
J. Org. Chem. 72(15) , 5759-70, (2007) Chiral acid chlorides were reacted with isoquinoline and 6,7-dimethoxy-3,4-dihydroisoquinoline to form diastereomeric Reissert compounds 8-11 and 18-21, respectively. The best diastereoselectivity (80... |
| 2,2-Dimethylpropanal |
| α,α-Dimethylpropanal |
| Pivalaldehyde |
| Propanal, 2,2-dimethyl- |
| trimethylacetaldehyde |
| 2,2-Dimethylpropionaldehyde |
| Pivalic aldehyde |
| EINECS 211-134-6 |
| MFCD00006962 |
| α,α-Dimethylpropionaldehyde |
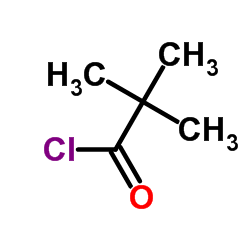 CAS#:3282-30-2
CAS#:3282-30-2 CAS#:75-98-9
CAS#:75-98-9 CAS#:75-84-3
CAS#:75-84-3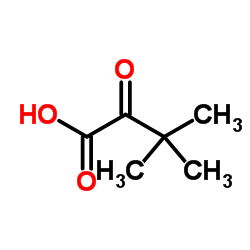 CAS#:815-17-8
CAS#:815-17-8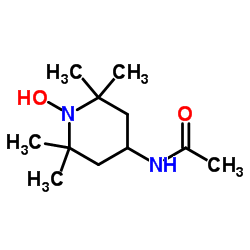 CAS#:14691-89-5
CAS#:14691-89-5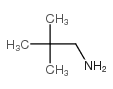 CAS#:5813-64-9
CAS#:5813-64-9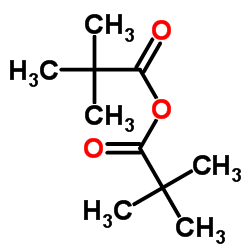 CAS#:1538-75-6
CAS#:1538-75-6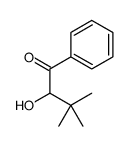 CAS#:56346-02-2
CAS#:56346-02-2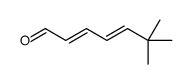 CAS#:101415-87-6
CAS#:101415-87-6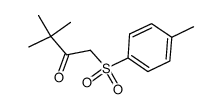 CAS#:101268-22-8
CAS#:101268-22-8 CAS#:598-98-1
CAS#:598-98-1 CAS#:492-41-1
CAS#:492-41-1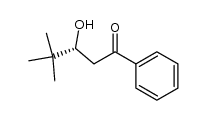 CAS#:118888-18-9
CAS#:118888-18-9 CAS#:504-61-0
CAS#:504-61-0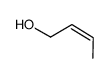 CAS#:4088-60-2
CAS#:4088-60-2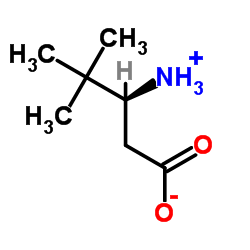 CAS#:367278-48-6
CAS#:367278-48-6
