TRIMETHYLACETIC ANHYDRIDE
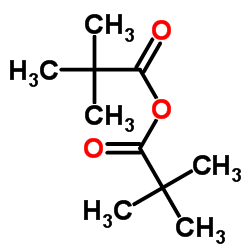
TRIMETHYLACETIC ANHYDRIDE structure
|
Common Name | TRIMETHYLACETIC ANHYDRIDE | ||
|---|---|---|---|---|
| CAS Number | 1538-75-6 | Molecular Weight | 186.248 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 193.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H18O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 57.2±0.0 °C | |
| Symbol |


GHS02, GHS05 |
Signal Word | Danger | |
| Name | Trimethylacetic anhydride |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 193.0±0.0 °C at 760 mmHg |
| Molecular Formula | C10H18O3 |
| Molecular Weight | 186.248 |
| Flash Point | 57.2±0.0 °C |
| Exact Mass | 186.125595 |
| PSA | 43.37000 |
| LogP | 1.97 |
| Vapour Pressure | 0.5±0.3 mmHg at 25°C |
| Index of Refraction | 1.429 |
| Storage condition | Store at RT. |
| Symbol |


GHS02, GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H226-H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | C:Corrosive; |
| Risk Phrases | R34 |
| Safety Phrases | S26-S36/37/39-S45 |
| RIDADR | UN 2920 8/PG 2 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 8 |
| HS Code | 2942000000 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2915900090 |
|---|---|
| Summary | 2915900090 other saturated acyclic monocarboxylic acids and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:5.5% General tariff:30.0% |
|
Kinetic resolution of racemic 2-hydroxy-γ-butyrolactones by asymmetric esterification using diphenylacetic acid with pivalic anhydride and a chiral acyl-transfer catalyst.
Org. Lett. 15(6) , 1170-3, (2013) Various optically active 2-hydroxy-γ-butyrolactone derivatives are produced via the kinetic resolution of racemic 2-hydroxy-γ-butyrolactones with diphenylacetic acid using pivalic anhydride and (R)-be... |
|
|
The concept of superactive esters. Could peptide synthesis be improved by inventing superactive esters?
Int. J. Pept. Protein Res. 43 , 312, (1994) According to the concept presented, esters forming an amide (peptide) bond by the mechanism SN#DN or SN#*DN involving fast decay of the tetrahedral intermediate may behave as 'superactive acylating re... |
|
|
Observation and elimination of N-acetylation of oligonucleotides prepared using fast-deprotecting phosphoramidites and ultra-mild deprotection.
Bioorg. Med. Chem. Lett. 11(9) , 1105-1107, (2001) Commercially available 'fast-deprotecting' phosphoramidites are useful for synthesizing oligonucleotides containing alkali-sensitive nucleotides. However, N-acetylated oligonucleotides were observed d... |
| Pivalic anhydride |
| MFCD00008842 |
| 2,2-dimethylpropanoyl 2,2-dimethylpropanoate |
| Trimethylacetic Anhydride |
| 2,2-Dimethylpropionic Anhydride |
| 2,2-Dimethylpropanoic anhydride |
| 2,2-Dimethylpropionic anhydride,Pivalic anhydride |
| EINECS 216-263-1 |
| PROPANOIC ACID, 2,2-DIMETHYL-, ANHYDRIDE |
 CAS#:3282-30-2
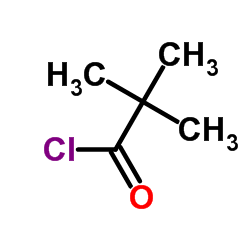
CAS#:3282-30-2 CAS#:75-98-9
CAS#:75-98-9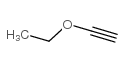 CAS#:927-80-0
CAS#:927-80-0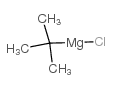 CAS#:677-22-5
CAS#:677-22-5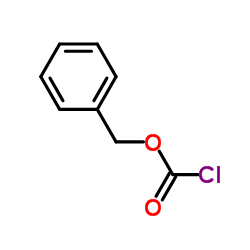 CAS#:501-53-1
CAS#:501-53-1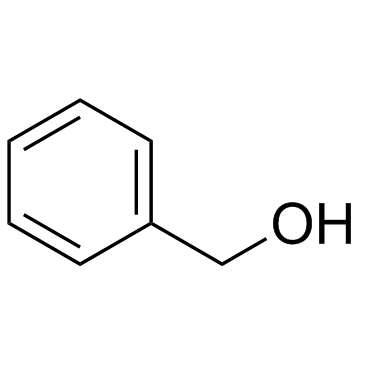 CAS#:100-51-6
CAS#:100-51-6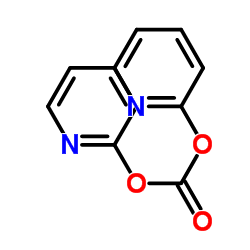 CAS#:1659-31-0
CAS#:1659-31-0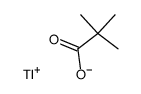 CAS#:80057-39-2
CAS#:80057-39-2 CAS#:35373-26-3
CAS#:35373-26-3 CAS#:1344680-35-8
CAS#:1344680-35-8 CAS#:38222-83-2
CAS#:38222-83-2 CAS#:108154-12-7
CAS#:108154-12-7 CAS#:108154-13-8
CAS#:108154-13-8 CAS#:51644-96-3
CAS#:51644-96-3 CAS#:39919-69-2
CAS#:39919-69-2 CAS#:91419-52-2
CAS#:91419-52-2 CAS#:5176-27-2
CAS#:5176-27-2 CAS#:4195-17-9
CAS#:4195-17-9
