Bioorganic & Medicinal Chemistry Letters
2001-05-07
Observation and elimination of N-acetylation of oligonucleotides prepared using fast-deprotecting phosphoramidites and ultra-mild deprotection.
Q Zhu, M O Delaney, M M Greenberg
Index: Bioorg. Med. Chem. Lett. 11(9) , 1105-1107, (2001)
Full Text: HTML
Abstract
Commercially available 'fast-deprotecting' phosphoramidites are useful for synthesizing oligonucleotides containing alkali-sensitive nucleotides. However, N-acetylated oligonucleotides were observed during solid-phase synthesis using 'fast-deprotecting' phosphoramidites in conjunction with K2CO3/MeOH ('ultra-mild') deprotection. Transamidation was localized at deoxyguanosine, which is protected as its isopropylphenoxyacetyl amide. Substitution of trimethylacetic anhydride for acetic anhydride and appropriate modification of the automated synthesis cycles eliminated this problem.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
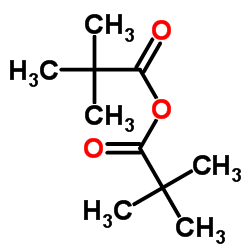 |
TRIMETHYLACETIC ANHYDRIDE
CAS:1538-75-6 |
C10H18O3 |
Related Articles:
More...
|
Kinetic resolution of racemic 2-hydroxy-γ-butyrolactones by ...
2013-03-15 [Org. Lett. 15(6) , 1170-3, (2013)] |
|
The concept of superactive esters. Could peptide synthesis b...
1994-03-01 [Int. J. Pept. Protein Res. 43 , 312, (1994)] |
|
New synthetic substrates of mammalian nucleotide excision re...
2013-07-01 [Nucleic Acids Res. 41 , e123, (2013)] |
|
Structural Basis for Substrate Specificity in Adenosylcobala...
2015-11-06 [J. Biol. Chem. 290 , 26882-98, (2015)] |
|
Kinetic resolution of the racemic 2-hydroxyalkanoates using ...
2010-01-04 [Chemistry 16(1) , 167-72, (2010)] |