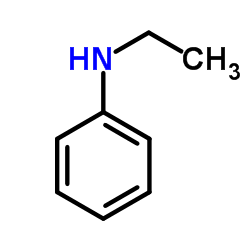
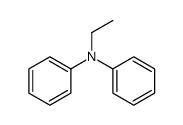
|
~0% |
|
~76% |
|
~9% |
|
~89% |
|
~86% |
|
~27% |
|
~83% |
|
~80% |
|
~% |
|
~60% |
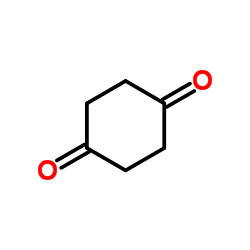
![1,4-Dioxaspiro[4.5]decan-8-one Structure](https://image.chemsrc.com/caspic/201/4746-97-8.png)

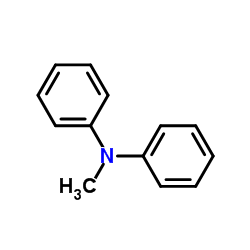
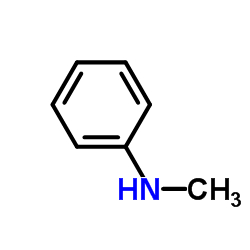
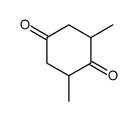
![7,9-dimethyl-1,4-dioxa-spiro[4.5]decan-8-one Structure](https://image.chemsrc.com/caspic/349/92115-20-3.png)
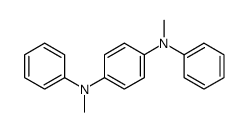


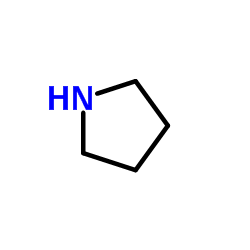
![1-(1,4-dioxaspiro[4.5]dec-7-en-8-yl)pyrrolidine Structure](https://image.chemsrc.com/caspic/444/57440-57-0.png)
![ethyl 8-oxo-1,4-dioxaspiro[4.5]decane-7-carboxylate Structure](https://image.chemsrc.com/caspic/086/14160-65-7.png)