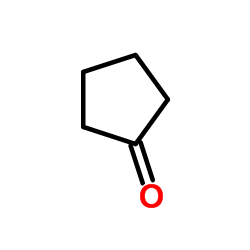
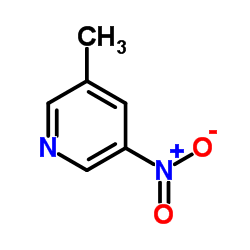
|
~23%
Detail
|
|
~45% |
|
~18% |
|
~62% |
|
~92% |
|
~79% |
|
~36% |
|
~37% |
|
~27% |
|
~32% |
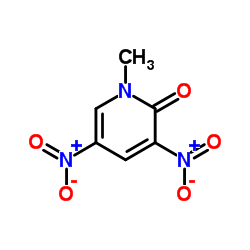
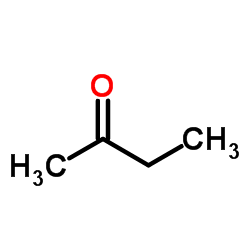
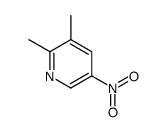
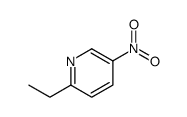
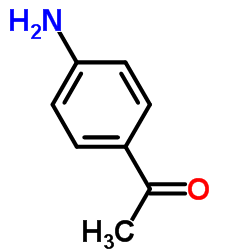
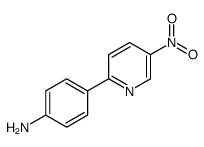
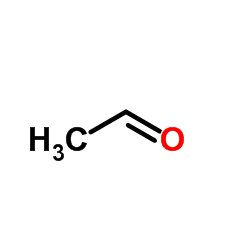
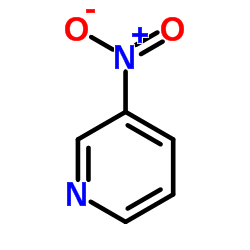
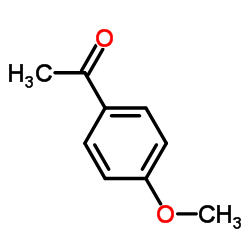
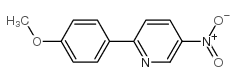
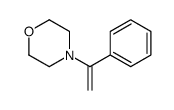
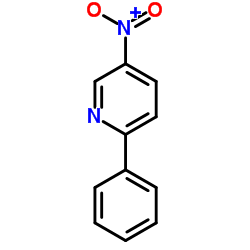


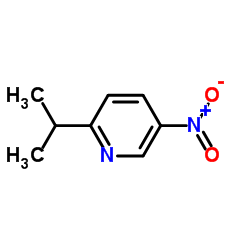

![3-nitro-6,7-dihydro-5h-cyclopenta[b]pyridine Structure](https://image.chemsrc.com/caspic/286/84531-36-2.png)